Abstract
Cell shape is controlled by the submembranous cortex, an actomyosin network mainly generated by two actin nucleators: the Arp2/3 complex and the formin mDia1. Changes in relative nucleator activity may alter cortical organization, mechanics and cell shape. Here we investigate how nucleation-promoting factors mediate interactions between nucleators. In vitro, the nucleation-promoting factor SPIN90 promotes formation of unbranched filaments by Arp2/3, a process thought to provide the initial filament for generation of dendritic networks. Paradoxically, in cells, SPIN90 appears to favour a formin-dominated cortex. Our in vitro experiments reveal that this feature stems mainly from two mechanisms: efficient recruitment of mDia1 to SPIN90–Arp2/3 nucleated filaments and formation of a ternary SPIN90–Arp2/3–mDia1 complex that greatly enhances filament nucleation. Both mechanisms yield rapidly elongating filaments with mDia1 at their barbed ends and SPIN90–Arp2/3 at their pointed ends. Thus, in networks, SPIN90 lowers branching densities and increases the proportion of long filaments elongated by mDia1.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Mass spectrometry data have been deposited in ProteomeXchange with the primary accession code PXD018318. All data supporting the conclusions of this paper are available from the authors upon reasonable request.
Code availability
The code used to analyse data in this study is available from the authors upon request.
References
Salbreux, G., Charras, G. & Paluch, E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 22, 536–545 (2012).
Ramanathan, S. P. et al. Cdk1-dependent mitotic enrichment of cortical myosin II promotes cell rounding against confinement. Nat. Cell Biol. 17, 148–159 (2015).
Chugh, P. et al. Actin cortex architecture regulates cell surface tension. Nat. Cell Biol. 19, 689–697 (2017).
Ennomani, H. et al. Architecture and connectivity govern actin network contractility. Curr. Biol. 26, 616–626 (2016).
Kovar, D. R. & Pollard, T. D. Progressing actin: formin as a processive elongation machine. Nat. Cell Biol. 6, 1158–1159 (2004).
Romero, S. et al. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 119, 419–429 (2004).
Fritzsche, M., Erlenkamper, C., Moeendarbary, E., Charras, G. & Kruse, K. Actin kinetics shapes cortical network structure and mechanics. Sci. Adv. 2, e1501337 (2016).
Acharya, B. R. et al. Mammalian diaphanous 1 mediates a pathway for e-cadherin to stabilize epithelial barriers through junctional contractility. Cell Rep. 18, 2854–2867 (2017).
Bovellan, M. et al. Cellular control of cortical actin nucleation. Curr. Biol. 24, 1628–1635 (2014).
May, R. C., Caron, E., Hall, A. & Machesky, L. M. Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat. Cell Biol. 2, 246–248 (2000).
Ridley, A. J. Life at the leading edge. Cell 145, 1012–1022 (2011).
Seth, A., Otomo, C. & Rosen, M. K. Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLα and mDia1. J. Cell Biol. 174 (2006).
Michelot, A. & Drubin, D. G. Building distinct actin filament networks in a common cytoplasm. Curr. Biol. 21, R560–569 (2011).
Murrell, M., Oakes, P. W., Lenz, M. & Gardel, M. L. Forcing cells into shape: the mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 16, 486–498 (2015).
Okada, K. et al. Adenomatous polyposis coli protein nucleates actin assembly and synergizes with the formin mDia1. J. Cell Biol. 189, 1087–1096 (2010).
Quinlan, M. E., Hilgert, S., Bedrossian, A., Mullins, R. D. & Kerkhoff, E. Regulatory interactions between two actin nucleators, Spire and Cappuccino. J. Cell Biol. 179, 117–128 (2007).
Montaville, P. et al. Spire and Formin 2 synergize and antagonize in regulating actin assembly in meiosis by a ping-pong mechanism. PLoS Biol. 12, e1001795 (2014).
Block, J. et al. FMNL2 drives actin-based protrusion and migration downstream of Cdc42. Curr. Biol. 22, 1005–1012 (2012).
Campellone, K. G. & Welch, M. D. A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 11, 237–251 (2010).
Brandt, D. T. et al. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J. Cell Biol. 178, 193–200 (2007).
Foroutannejad, S., Rohner, N., Reimer, M., Kwon, G. & Schober, J. M. A novel role for IQGAP1 protein in cell motility through cell retraction. Biochem Biophys. Res Commun. 448, 39–44 (2014).
Gorman, J. A. et al. The cytoskeletal adaptor protein IQGAP1 regulates TCR-mediated signaling and filamentous actin dynamics. J. Immunol. 188, 6135–6144 (2012).
Wagner, A. R., Luan, Q., Liu, S. L. & Nolen, B. J. Dip1 defines a class of Arp2/3 complex activators that function without preformed actin filaments. Curr. Biol. 23, 1990–1998 (2013).
Balzer, C. J., Wagner, A. R., Helgeson, L. A. & Nolen, B. J. Dip1 co-opts features of branching nucleation to create linear actin filaments that activate WASP-bound Arp2/3 complex. Curr. Biol. 28, 3886–3891 (2018).
Balzer, C. J., Wagner, A. R., Helgeson, L. A. & Nolen, B. J. Single-turnover activation of Arp2/3 complex by Dip1 . Curr. Biol. 29, 3331–3338 (2019).
Eisenmann, K. M. et al. Dia-interacting protein modulates formin-mediated actin assembly at the cell cortex. Curr. Biol. 17, 579–591 (2007).
Satoh, S. & Tominaga, T. mDia-interacting protein acts downstream of Rho–mDia and modifies Src activation and stress fiber formation. J. Biol. Chem. 276 (2001).
Higashi, T. et al. Flightless-I (Fli-I) regulates the actin assembly activity of diaphanous-related formins (DRFs) Daam1 and mDia1 in cooperation with active Rho GTPase. J. Biol. Chem. 285, 16231–16238 (2010).
Dephoure, N. et al. A quantitative atlas of mitotic phosphorylation. Proc. Natl Acad. Sci. USA 105, 10762–10767 (2008).
Olsen, J. V. et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci. Signal 3, ra3 (2010).
Bekker-Jensen, D. B. et al. An optimized shotgun strategy for the rapid generation of comprehensive human proteomes. Cell Syst. 4, 587–599 (2017).
Charras, G. T., Hu, C. K., Coughlin, M. & Mitchison, T. J. Reassembly of contractile actin cortex in cell blebs. J. Cell Biol. 175, 477–490 (2006).
Clark, A. G., Dierkes, K. & Paluch, E. K. Monitoring actin cortex thickness in live cells. Biophys. J. 105, 570–580 (2013).
Biro, M. et al. Cell cortex composition and homeostasis resolved by integrating proteomics and quantitative imaging. Cytoskeleton 70, 741–754 (2013).
Vargas-Pinto, R., Gong, H., Vahabikashi, A. & Johnson, M. The effect of the endothelial cell cortex on atomic force microscopy measurements. Biophys. J. 105, 300–309 (2013).
Bergert, M., Chandradoss, S. D., Desai, R. A. & Paluch, E. Cell mechanics control rapid transitions between blebs and lamellipodia during migration. Proc. Natl Acad. Sci. USA 109, 14434–14439 (2012).
Toyoda, Y. et al. Genome-scale single-cell mechanical phenotyping reveals disease-related genes involved in mitotic rounding. Nat. Commun. 8, 1266 (2017).
Sullivan-Brown, J. L. et al. Identifying regulators of morphogenesis common to vertebrate neural tube closure and Caenorhabditis elegans gastrulation. Genetics 202, 123–139 (2016).
Sokol, S. Y., Li, Z. & Sacks, D. B. The effect of IQGAP1 on Xenopus embryonic ectoderm requires Cdc42. J. Biol. Chem. 276, 48425–48430 (2001).
Higashi, T., Stephenson, R. E. & Miller, A. L. Comprehensive analysis of formin localization in Xenopus epithelial cells. Mol. Biol. Cell 30, 82–95 (2019).
Jones, E. A. & Woodland, H. R. Development of the ectoderm in Xenopus: tissue specification and the role of cell association and division. Cell 44, 345–355 (1986).
Jurgenson, C. T. & Pollard, T. D. Crystals of the Arp2/3 complex in two new space groups with structural information about actin-related protein 2 and potential WASP binding sites. Acta Crystallogr F. 71, 1161–1168 (2015).
Luan, Q., Liu, S. L., Helgeson, L. A. & Nolen, B. J. Structure of the nucleation-promoting factor SPIN90 bound to the actin filament nucleator Arp2/3 complex. EMBO J. 37, e100005 (2018).
Cao, L. et al. Modulation of formin processivity by profilin and mechanical tension. eLife 7, e34176 (2018).
Rotty, J. D. & Bear, J. E. Competition and collaboration between different actin assembly pathways allows for homeostatic control of the actin cytoskeleton. Bioarchitecture 5, 27–34 (2014).
Suarez, C. et al. Profilin regulates F-actin network homeostasis by favoring formin over Arp2/3 complex. Dev. Cell 32, 43–53 (2015).
Bombardier, J. P. et al. Single-molecule visualization of a formin-capping protein ‘decision complex’ at the actin filament barbed end. Nat. Commun. 6, 8707 (2015).
Breitsprecher, D. et al. Rocket launcher mechanism of collaborative actin assembly defined by single-molecule imaging. Science 336, 1164–1168 (2012).
Funk, J. et al. Profilin and formin constitute a pacemaker system for robust actin filament growth. eLife 8, e50963 (2019).
Cunningham, C. C. et al. Actin-binding protein requirement for cortical stability and efficient locomotion. Science 255, 325–327 (1992).
Ren, J. G., Li, Z., Crimmins, D. L. & Sacks, D. B. Self-association of IQGAP1: characterization and functional sequelae. J. Biol. Chem. 280, 34548–34557 (2005).
Seward, M. E., Easley, C. A. IV, McLeod, J. J., Myers, A. L. & Tombes, R. M. Flightless-I, a gelsolin family member and transcriptional regulator, preferentially binds directly to activated cytosolic CaMK-II. FEBS Lett. 582, 2489–2495 (2008).
Ai, H. W., Shaner, N. C., Cheng, Z., Tsien, R. Y. & Campbell, R. E. Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins. Biochemistry 46, 5904–5910 (2007).
Svitkina, T. M. & Borisy, G. G. Correlative light and electron microscopy of the cytoskeleton of cultured cells. Methods Enzymol. 298, 570–592 (1998).
Tinevez, J. Y. et al. Role of cortical tension in bleb growth. Proc. Natl Acad. Sci. USA 106, 18581–18586 (2009).
Crick, S. L. & Yin, F. C. Assessing micromechanical properties of cells with atomic force microscopy: importance of the contact point. Biomech. Model Mechanobiol. 6, 199–210 (2007).
Sneddon, I. N. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 3, 10 (1965).
Mindell, J. A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003).
de la Rosa-Trevin, J. M. et al. Scipion: A software framework toward integration, reproducibility and validation in 3D electron microscopy. J. Struct. Biol. 195, 93–99 (2016).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Otomo, T. et al. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature 433, 488–494 (2005).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput Chem. 25, 1605–1612 (2004).
Jegou, A., Carlier, M. F. & Romet-Lemonne, G. Microfluidics pushes forward microscopy analysis of actin dynamics. Bioarchitecture 1, 271–276 (2011).
Acknowledgements
This article is dedicated to the memory of Art Alberts who passed away during completion of this study. We thank present and past members of the Charras, Romet/Jegou, Paluch and Roux labs for support and discussions over the course of the project; A. Gautreau and A. Yap for critical reading of the draft manuscript; and H. Higgs, R. Grosse, B. Nolen, D. Sacks, R. Tombes and O. Weiner for sharing reagents. A.Y., M.V., P.C. and M.B. were supported by Human Frontier Science Program Young Investigator grants to G.C., E.K.P., G.R.-L. and P.P.R. (RGY66/2013). P.C. and E.K.P. acknowledge the support of the Medical Research Council UK (Medical Research Council programme award MC_UU_12018/5). E.F. was funded by a European Research Council consolidator grant to G.C. (CoG-647186). E.H.B. was supported by a Biotechnology and Biological Sciences Research Council grant (BB/R00627) to G.C. and R.M. L.C. was supported by a grant from Fondation pour la Recherche Médicale (DEI 20151234415) to G.R.-L. J.M. was supported by a grant from the Agence Nationale de la Recherche to A.B. and G.R.-L. (Agence Nationale de la Recherche grant Conformin). A.J. was supported by a European Research Council starting grant (StG-679116). AFM equipment was funded by a Biotechnology and Biological Sciences Research Council Alert16 grant to G.C. (BB/R000042). A.M. and G.L. were supported by an Operating Grant from the Canadian Institute of Health Research to P.P.R. (MOP‑142374). P.P.R. is supported by a The Fonds de recherche du Québec–Santé Senior Investigator Career Award. We acknowledge the Cell and Tissue Imaging (PICT IBiSA, Institut Curie) platform supported by France-BioImaging (ANR10-INBS-04) for the electron microscopy equipment.
Author information
Authors and Affiliations
Contributions
G.C., G.R.-L., A.J., A.Y., L.C. and M.V. designed the experiments. A.Y. carried out all scanning electron microscopy on cells, long-term microscopy and bleb-size characterization. M.V. carried out all laser ablation, AFM and fluorescence-quantification experiments. L.C., G.R.-L. and A.J. carried out in vitro experiments. P.C. did all measurements of cortex thickness and F-actin density. M.B.S. designed the software for analysis of SEM images. G.L., A.M. and P.P.R. performed all proteomic and quantitative PCR experiments and analysed data. A.Y. and E.F. carried out all western blotting. G.C. generated plasmid constructs and cell lines and carried out immunostaining. J.M. and A.B. carried out the molecular-scale electron microscopy and generated the complex structures. M.B. contributed live-imaging constructs and some imaging experiments. A.A. provided plasmid constructs. E.H.B. and R.M. contributed Xenopus experiments. E.K.P. provided conceptual advice. G.C. and G.R.L. wrote the manuscript. All authors discussed the results and the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Localisation of NPFs in blebbing melanoma cells.
(a-d) Confocal microscopy images of blebbing M2 melanoma cells expressing GFP-tagged NPFs. White arrowheads indicate expanding blebs and red arrowheads retracting ones. Scale bars=5μm. Experiments were repeated two times independently with similar results. a, IQGAP1 localizes to the rim of retracting blebs (red arrowhead) but not to the rim of nascent blebs (white arrowhead). b, SPIN90-GFP before (left) and after (right) permeabilization and fixation to remove cytoplasmic signal. Following permeabilization and fixation, SPIN90 remains localized to the actin cortex of retracting blebs but is absent from the cortex of nascent blebs. c, FLII-GFP before (left) and after (right) permeabilization and fixation. Following permeabilization and fixation, FLII remains localized to the actin cortex of retracting blebs. d, Constitutively active cortactin-GFP localizes to the rim of retracting blebs but not to the rim of nascent blebs.
Extended Data Fig. 2 Control experiments for actin accumulation rate and cortical stiffness.
a, Representative laser ablation experiment. All images are a single confocal section. Top row: Alexa647 is added to the medium for robust segmentation of the cell. Bottom row: LifeAct Ruby. Left column: cell before ablation, middle column: after ablation, right column: segmented image. Timings are indicated on the top row images. Scale bar=10 µm. b, Representative actin regrowth curve in a bleb induced by laser ablation as in A. Pink curve: evolution of mean cortical actin fluorescence in the bleb normalized to the mean cortical intensity in the cell body (pink). Blue curve: evolution of bleb area normalized to cell body area. Initial regrowth rates are linear with time (initial accumulation rate). t = 0 s, ablation onset. (c-e) Data plotted as box-whisker plots. The distributions’ medians, first and third quartiles, and ranges are represented by the central red bars, bounding boxes and whiskers, respectively. Statistics are derived from the total number (n, indicated above each box) of cells examined in three independent experiments. Each dot represents one cell measurement. Statistical outliers are indicated by red dots. **p < 0.01 compared to the appropriate control. c, Actin accumulation rate in metaphase cells at 37 C and Room Temperature. Two-sided Student t-test: 37 C vs RT: p = 0.19. d, Apparent elastic modulus for cells expressing Non-Silencing shRNA (NS sh), transfected with Non-Silencing siRNA (NS si), and treated with DMSO. One-way ANOVA on ranks: NS siRNA vs NS shRNA: p = 0.001, DMSO vs NS shRNA: p = 0.001. DMSO vs NS siRNA: p = 0.99. e, Apparent elastic modulus of WT and ACTR2 shRNA cells treated with DMSO and blebbistatin (Bb). One-way ANOVA on ranks: WT DMSO vs WT blebbistatin: p = 2 10-9; WT DMSO vs ACTR2 shRNA DMSO: p = 4 10-5; ACTR2 shRNA DMSO vs ACTR2 shRNA blebbistatin: p = 5 10-13; WT blebbistatin vs ACTR2 shRNA blebbistatin: p = 0.71. Statistical source data can be found at Source data figure ED2.
Extended Data Fig. 3 Regulation of myosin localization and phosphorylation in cells with nucleator or NPF depletion.
a, Representative pMLC distribution in metaphase HeLa cells visualized by immuno-staining for different protein depletions and for non-silencing (NS) siRNA. Each image is a single section of a confocal microscopy stack and is shown in inverted contrast. b, Mean cortical pMLC fluorescence intensity for different treatments normalized to the mean cortical pMLC fluorescence intensity for non-silencing (NS) siRNA or shRNA. The distributions’ medians, first and third quartiles and ranges are represented by the central red bars, bounding boxes and whiskers, respectively. Statistics are derived from the total number (n, indicated above each box) of cells examined in three independent experiments. Each dot represents one cell measurement. Statistical outliers are indicated by red dots. Statistical comparisons of the means were performed using one-way ANOVA on ranks compared to NS siRNA/shRNA. ACTR2 siRNA: p = 7 10-5, NAP1: p = 0.03, mDia1: p = 0.85, IQGAP1: p = 0.08, SPIN90: p = 0.74. **p < 0.01 compared to the appropriate control. See Supplementary Figure 9 for controls. c, Change in cortical myosin regulatory light chain fluorescence intensity upon treatment with DMSO (left panel) or CK666 (right panel). In each panel, the top row shows the fluorescence intensity before treatment and the bottom row after treatment for the same cell. The left most column shows myosin regulatory light chain fluorescence (MRLC-GFP), the middle column shows LifeAct-Ruby, and the right column shows the overlay with MRLC in green and LifeAct in Magenta. Experiments were repeated twice independently with similar results. (a,c) Scale bars=10 µm. Statistical source data can be found at Source data figure ED3.
Extended Data Fig. 4 Interaction of SPIN90 with single nucleators.
a, Sketch of a microfluidics experiment, where surface-anchored SPIN90 is exposed to 30 nM Arp2/3 complex for 5 min, followed by profilin-actin (15% Alexa488 labeled). TIRF microscope image: filaments nucleate and grow from their free barbed ends while their pointed ends remain attached. Plot: appearance of 217 filaments over time (red dots), with an estimated nucleation rate of 9.5×10-4 s-1 (exponential fit, solid line). b, Microfluidics experiment where surface-anchored SPIN90 is exposed to 50 nM mDia1 for 5 min, followed by profilin-actin (15% Alexa488 labeled) and then profilin-actin (unlabeled). Kymograph (from TIRF microscopy): filaments grow rapidly from their anchored barbed ends. Plot: elongation rate of these filaments (blue, n = 17 measured filaments) compared to filaments growing from mDia1 directly anchored to the surface without SPIN90 (red, n = 17 measured filaments). Boxes indicate averages and standard deviation, for each group of n filaments. c, GST pull down assay to detect binding between mDia1 and SPIN90. GST or GST-fused mDia1 coated beads were incubated with various concentrations of His tag-fused SPIN90 for 1 hour. Proteins attached to the beads were eluted with 20 mM GSH. The presence of SPIN90 was detected by western blot. The experiments were repeated three times independently with similar results. Uncropped Western blot can be found in Source data figure ED4. d, Density of rapidly growing filament barbed ends (i.e. bearing a formin), observed over 74752 μm2 after 300 s in the experiment shown in Fig. 5C. The error bars show the 95% confidence interval. Statistical source data can be found at Source data figure ED4.
Extended Data Fig. 5 mDia1 preferentially binds to filaments nucleated by SPIN90-Arp2/3.
a, Comparison of mDia1 binding to mother filaments versus branches. Preformed branched actin filaments (15% Alexa568 labeled) were mixed with 0.5 μM G-actin (15% Alexa488-labeled), 0.5 μM profilin, 0.4 nM mDia1, before being introduced into a passivated open chamber. The TIRF microscopy image shows Alexa568 in red and Alexa488 in green. Plot: fraction of rapidly growing barbed ends (i.e. bearing a formin) observed after 90 s, within the population of mother filaments (dark blue bar) and branches (light blue bar). Comparison with Pearson’s chi square test: p = 0.88. b, Comparison of mDia1 binding to spontaneously nucleated versus SPIN90-Arp2/3-nucleated filaments. Spontaneously nucleated actin filaments (15% Alexa568-labeled) were mixed with SPIN90-Arp2/3-nucleated actin filaments (15% Alexa488-labeled) and with 0.2 nM mDia1, 0.5 µM profilin and 0.5 µM G-actin (3% Alexa488-labeled). TIRF microscopy image: filaments growing from spontaneously assembled seeds (red arrow) or from SPIN90-Arp2/3-nucleated seeds (green arrow) are identified by the fluorescence at their pointed end. Plot: fraction of rapidly growing barbed ends observed after 300 s, within each population. Comparison with a Pearson’s chi square test: p = 0.009. Similar results were observed when repeating the experiment and inverting the fluorophores (Supplementary Figure 8B). c, Comparison of the fraction of mDia1-bearing barbed ends depending on filament nucleation history. SPIN90-Arp2/3-nucleated actin filaments (15% Alexa568 labeled) were mixed with 0.5 μM G-actin (15% Alexa488 labeled), 0.5 μM profilin, 25 nM Arp2/3, 250 nM SPIN90 and 0.2 nM mDia1. TIRF microscopy image: filaments nucleated before mixing (i.e. exposed to mDia1 after their nucleation by SPIN90-Arp2/3) are identified thanks to their Alexa568 (red) pointed end region. Plot: fraction of rapidly growing barbed ends observed after 300 s, within each population. Comparison with a Pearson’s chi square test: p = 9×10-7. (a,b,c) Error bars represent 95% confidence intervals. **p < 0.01 and ***p < 0.001, Pearson’s chi-squared test. For each population, n indicates the number of analysed barbed ends, in one experiment. The experiments (A, B) were repeated twice, and (C) three times, independently with similar results. Statistical source data can be found at Source data figure ED5.
Extended Data Fig. 6 mDia1 binds to the SPIN90-Arp2/3 complex.
a, mDia1 is dose-dependently eluted with Arp2/3 bound to GST-SPIN90 decorated beads. Left: Anti-His and Anti-ArpC2 western blots of GST pull down assay. GST beads were incubated with 12 μM GST-SPIN90 and with the indicated amounts of Arp2/3. The beads were washed and incubated with 750 nM mDia1. Right: quantification of pulled-down mDia1, normalized to GST-SPIN90 without Arp2/3 (mean ± SD, n = 3 independent experiments). Black dots show the data points. For each concentration, the red dot shows the mean and the red bars the standard deviation. Uncropped Western blot can be found in Source data figure ED6. b, Left: schematic diagram of a control experiment (related to Fig. 6C-D). Control experiments consist in observing the elongation of new, bare barbed ends growing in a microfluidics chamber with a SPIN90-decorated surface exposed to Arp2/3 and mDia1. This is achieved by first following the sequence shown in Fig. 6C, and then photo-severing the filaments with a strong laser illumination (see Methods). We then observed the regrowth of filaments from SPIN90-Arp2/3. The photo-severing ensured that the newly elongating barbed ends were initially without mDia1. The distribution of slow (light blue), slow then fast (blue), and fast (dark blue) filaments was compared to experiments like the ones presented in Fig. 6C-D (bar charts, on the right, n=number of filaments). “Complex” denotes the experiments performed as in Fig. 6C-D (with an additional exposure to light, to have the same conditions as in the control, see Methods) and “control” denotes the control experiments. Comparison using one-tailed Fisher’s exact test: p = 0.31 for the slow-then-fast population, and p = 0.0013 for the fast population. n is the number of filaments randomly picked and investigated in an independent experiment. The experiment was repeated twice independently with similar results. Statistical source data can be found at Source data figure ED6.
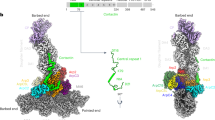
Extended Data Fig. 7 Visualisation of different orientations of the protein complexes formed by Arp2/3, SPIN90, and mDia1.
Different combinations of complexes (binary or ternary) involving the Arp2/3 complex, SPIN90, and mDia1 were analyzed by electron microscopy and single particle analysis. For each protein complex, three-dimensional reconstructions were obtained from 3D classifications and compared to existing or generated crystal structures (see methods). a, Incubation of mDia1 and Arp2/3 resulted in a 3D structure which only accommodates Arp2/3, suggesting that mDia1 and Arp2/3 do not interact when SPIN90 is absent. b, Conversely, mixing the Arp2/3 complex and SPIN90 results in a complex for 76 % of the particles. Within the complex, SPIN90 (green) clearly appears as an additional density when compared with the truncated docked crystal structure. c, When compared with the Arp2/3-SPIN90 complex in B, the 3D envelope (resolution of 27 Å) resulting from the ternary complex (SPIN90-Arp2/3-mDia1) exhibits an additional density accommodating a dimer of FH2 domains for 22 % of the particles. (a-c) Each protein complex is subjected to a variety of rotations to visualize its full structure.
Extended Data Fig. 8 Raw data of negatively stained Arp2/3, SPIN90, mDia1 mixes.
Scale bars: 100 nm in upper images, 15 nm in middle images; lower boxes are 29.8 nm2. a, Micrograph of Arp2/3 mixed with mDia1 (top), subselection of particles used for Arp2/3 3D reconstruction (middle), and examples of 2D classes (bottom). b, Micrograph of Arp2/3 mixed with SPIN90 (top), subselection of particles used for Arp2/3-SPIN90 3D reconstruction (middle), and examples of 2D classes (bottom). c, Micrograph of Arp2/3 with SPIN90 and mDia1 (top), subselection of particles used for Arp2/3-SPIN90-mDia1 3D reconstruction (middle), and examples of 2D classes (bottom).
Extended Data Fig. 9 Microfluidic experiments examining interactions of mDia1 with preformed filaments and with SPIN90-Arp2/3.
a, Sketch of microfluidics experiment related to Fig. 6A, showing how two groups of filaments generated by different nucleators were generated and identified. SPIN90 and spectrin-actin seeds were anchored to the surface (1st row), and exposed to 0.7 µM 15% Alexa488 G-actin to generate filaments nucleated from the seeds (2nd row). Then Arp2/3 was flowed in (3rd row), followed by 0.7 µM 15% Alexa488 G-actin to generate SPIN90-Arp2/3-nucleated filaments (4th row). Since all the spectrin-actin seeds had already nucleated filaments during the first exposure to actin, the newly nucleated filaments were identified as nucleated by SPIN90-Arp2/3. Finally, mDia1 was introduced into the chamber with profilin-actin (5th row). b, mDia1’s binding to filaments nucleated by spectrin-actin seeds with different lengths, related to Fig. 6A. To check whether mDia1 bound differently to filaments with different lengths, filaments nucleated by spectrin-actin seeds in a microfluidics setup, with heterogeneous lengths, were exposed to 0.4 nM mDia1, 0.5 μM 15% Alexa488 G-actin and 3.5 μM profilin. The binding of mDia1 to long filaments (>5 µm, n = 40 filaments) was compared to that for short filaments (<1 µm, n = 39 filaments), by plotting the percentage of fast-growing filaments in each population over time. The experiment was repeated twice independently, with similar results. Our results indicate no impact of filament length on mDia1 binding (p = 0.6, two sided log-rank test). Shaded regions, 95% confidence intervals. c, Sketch detailing the observation of the mDia1-free population reported in Fig. 6E. After the SPIN90-decorated surface was exposed to Arp2/3, we exposed only one half of the chamber to mDia1 (1). After rinsing with buffer (2), the surface was exposed to profilin-actin. In the region exposed to mDia1, filaments with different elongation rates appeared; while in the region that was not exposed to mDia1, only slow growing filaments were observed. Statistical source data can be found at Source data figure ED9.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13, Supplementary Results and Methods.
Supplementary Tables 1–8
Supplementary Table 1: NPFs detected in the detergent-insoluble fraction of blebs separated from M2 blebbing melanoma cells. Subunits of the same complex are displayed together. Protein isoforms are grouped together. Detected peptide numbers are averaged over three separate experiments. Protein abundance index (PAI) was calculated based on spectral counts (see Supplementary Methods). Supplementary Table 2: Copy number of proteins of interest per cell and copy number normalized to actin. Data from proteomic studies on HeLa cells by Bekker-Jensen et al31. For the Arp2/3 complex and the WAVE complex, the copy number of the least abundant subunit was taken as an estimate of complex abundance. Recent work has shown that the concentration of actin in a variety of cell lines is between 150 and 200 µM49. Assuming an actin concentration of 150 µM, this allows us to estimate the concentration of nucleators and NPFs: mDia1: 1.4 µM, Arp2/3 complex: 3.5 µM, WAVE complex: 500 nM, SPIN90: 250 nM, IQGAP1: 6.8 µM, Profilin 1: 61 µM. As a number of these proteins must be activated to play a part in nucleation and some are localized to the membrane while others are localized to the cytoplasm, the actual concentration of protein to include in in vitro assays is challenging to estimate. Supplementary Table 3: Graphic summary of experimental findings in cellular assays. The depletion target is indicated in the top row. The left hand side column indicates the assay considered, the cell type it was performed in, and the figure panel it relates to. All data is normalized to the value for the appropriate control. Each cell is color coded with blue indicating statistically significant decreases relative to control and red indicating statistically significant increases relative to control. The statistical tests utilized are described in the figure panel indicated in the left-most column. Supplementary Table 4: Raw data and statistical analysis of the phenotypes in the shRNA blebbing screen. Cells stably expressing the shRNA construct of interest were categorized as normal, displaying large blebs, and displaying small blebs. For each targeting shRNA construct, observed distributions were compared to expected numbers based on the categorization observed in non-silencing shRNA control using a chi-square test. Differences were considered significant for p < 0.01. Bleb size distributions were compared to those obtained for depletion of mDia1 and ACTR2 obtained in9. Supplementary Table 5: pGIPZ shRNA constructs. The columns of the table contain the name of the construct, accession number of the targeted gene, manufacturer’s oligo ID, and the mature sense sequence of each construct used in the shRNA. Supplementary Table 6: pLKO shRNA constructs (Human). The columns of the table contain the name of the construct, accession number of the targeted gene, manufacturer’s oligo ID, and the mature sense sequence of each construct used in the shRNA. Supplementary Table 7: siRNA ON-TARGETplus pool sequences (Human, GE Dharmacon), Diaph1 Stealth siRNA (Human, Thermo Fisher). The table indicates the name of the construct, the four target sequences contained in the pool, and the manufacturer’s oligo ID for each siRNA pool. Supplementary Table 8: List of primary antibodies used in Western blotting. Suppliers, dilution, and host species are indicated.
Source data
Statistical Source Data Fig. 1
Statistical source data
Statistical Source Data Fig. 2
Statistical source data
Statistical Source Data Fig. 3
Statistical source data
Statistical Source Data Fig. 4
Statistical source data
Statistical Source Data Fig. 5
Statistical source data
Statistical Source Data Fig. 6
Statistical source data
Statistical Source Data Extended Data Fig. 2
Statistical source data
Statistical source Data Extended Data Fig. 3
Statistical source data
Statistical source Data Extended Data Fig. 4
Statistical source data
Unprocessed Blots Extended Data Fig. 4
Unprocessed blot
Statistical source Data Extended Data Fig. 5
Statistical source data
Statistical source Data Extended Data Fig. 6
Statistical source data
Unprocessed Blots Extended Data Fig. 6
Unprocessed blot
Statistical source Data Extended Data Fig. 9
Statistical source data
Rights and permissions
About this article
Cite this article
Cao, L., Yonis, A., Vaghela, M. et al. SPIN90 associates with mDia1 and the Arp2/3 complex to regulate cortical actin organization. Nat Cell Biol 22, 803–814 (2020). https://doi.org/10.1038/s41556-020-0531-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-020-0531-y
This article is cited by
-
Adherens junctions as molecular regulators of emergent tissue mechanics
Nature Reviews Molecular Cell Biology (2024)
-
Regulation of the Scar/WAVE complex in migrating cells: A summary of our understanding
Journal of Biosciences (2023)
-
Biochemical and mechanical regulation of actin dynamics
Nature Reviews Molecular Cell Biology (2022)
-
F-actin architecture determines constraints on myosin thick filament motion
Nature Communications (2022)
-
Cell clusters softening triggers collective cell migration in vivo
Nature Materials (2022)