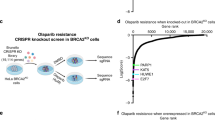
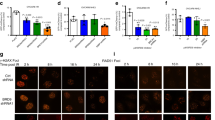
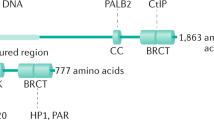
Abstract
The response to poly(ADP-ribose) polymerase inhibitors (PARPi) is dictated by homologous recombination (HR) DNA repair and the abundance of lesions that trap PARP enzymes. It remains unclear, however, if the established role of PARP in promoting chromatin accessibility impacts viability in these settings. Using a CRISPR-based screen, we identified the PAR-binding chromatin remodeller ALC1/CHD1L as a key determinant of PARPi toxicity in HR-deficient cells. ALC1 loss reduced viability of breast cancer gene (BRCA)-mutant cells and enhanced sensitivity to PARPi by up to 250-fold, while overcoming several resistance mechanisms. ALC1 deficiency reduced chromatin accessibility concomitant with a decrease in the association of base damage repair factors. This resulted in an accumulation of replication-associated DNA damage, increased PARP trapping and a reliance on HR. These findings establish PAR-dependent chromatin remodelling as a mechanistically distinct aspect of PARPi responses and therapeutic target in HR-deficient cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Sequencing data generated in this study have been deposited in the Gene Expression Omnibus with accession codes GSE149104 (for RNA-seq) and GSE150955 (for ATAC-seq). Data from the CRISPR screen have been provided as mapped reads in Supplementary Table 1. Functionally conserved domains were identified using either the NCBI Conserved Domain Search or UniProt. All other data supporting the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
All the analyses were based on standard algorithms described in the Methods and referenced accordingly. There are no custom algorithms to make available.
References
Langelier, M.-F., Planck, J. L., Roy, S. & Pascal, J. M. Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science 336, 728–732 (2012).
Langelier, M.-F., Riccio, A. A. & Pascal, J. M. PARP-2 and PARP-3 are selectively activated by 5′ phosphorylated DNA breaks through an allosteric regulatory mechanism shared with PARP-1. Nucleic Acids Res. 42, 7762–7775 (2014).
Gupte, R., Liu, Z. & Kraus, W. L. PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes Dev. 31, 101–126 (2017).
Poirier, G. G., de Murcia, G., Jongstra-Bilen, J., Niedergang, C. & Mandel, P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc. Natl Acad. Sci. USA 79, 3423–3427 (1982).
Krishnakumar, R. et al. Reciprocal binding of PARP-1 and histone H1 at promoters specifies transcriptional outcomes. Science 319, 819–821 (2008).
Hakmé, A., Wong, H.-K., Dantzer, F. & Schreiber, V. The expanding field of poly(ADP-ribosyl)ation reactions. ‘Protein modifications: beyond the usual suspects’ review series. EMBO Rep. 9, 1094–1100 (2008).
Pascal, J. M. The comings and goings of PARP-1 in response to DNA damage. DNA Repair 71, 177–182 (2018).
Luijsterburg, M. S. et al. PARP1 Links CHD2-mediated chromatin expansion and H3.3 deposition to DNA repair by non-homologous end-joining. Mol. Cell 61, 547–562 (2016).
Mateos-Gomez, P. A. et al. Mammalian polymerase θ promotes alternative NHEJ and suppresses recombination. Nature 518, 254–257 (2015).
Ronson, G. E. et al. PARP1 and PARP2 stabilise replication forks at base excision repair intermediates through Fbh1-dependent Rad51 regulation. Nat. Commun. 9, 746 (2018).
Ray Chaudhuri, A. et al. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat. Struct. Mol. Biol. 19, 417–423 (2012).
Fisher, A. E. O., Hochegger, H., Takeda, S. & Caldecott, K. W. Poly(ADP-ribose) polymerase 1 accelerates single-strand break repair in concert with poly(ADP-ribose) glycohydrolase. Mol. Cell. Biol. 27, 5597–5605 (2007).
Pines, A. et al. PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1. J. Cell Biol. 199, 235–249 (2012).
Bryant, H. E. et al. PARP is activated at stalled forks to mediate Mre11-dependent replication restart and recombination. EMBO J. 28, 2601–2615 (2009).
Golia, B., Singh, H. R. & Timinszky, G. Poly-ADP-ribosylation signaling during DNA damage repair. Front. Biosci. 20, 440–457 (2015).
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Helleday, T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol. Oncol. 5, 387–393 (2011).
Murai, J. et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 72, 5588–5599 (2012).
Gogola, E., Rottenberg, S. & Jonkers, J. Resistance to PARP inhibitors: lessons from preclinical models of BRCA-associated cancer. Annu. Rev. Cancer Biol. 3, 235–254 (2019).
Shi, J. et al. Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat. Biotechnol. 33, 661–667 (2015).
Najm, F. J. et al. Orthologous CRISPR-Cas9 enzymes for combinatorial genetic screens. Nat. Biotechnol. 36, 179–189 (2018).
Menear, K. A. et al. 4-[3-(4-Cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: a novel bioavailable inhibitor ofpoly(ADP-ribose) polymerase-1. J.Med. Chem. 51, 6581–6591 (2008).
Horton, J. K. et al. XRCC1 and DNA polymerase β in cellular protection against cytotoxic DNA single-strand breaks. Cell Res. 18, 48–63 (2008).
Lord, C. J. & Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 16, 110–120 (2016).
Xu, G. et al. REV7 counteracts DNA double-strand break resection and affects PARP inhibition. Nature 521, 541–544 (2015).
Bunting, S. F. et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 141, 243–254 (2010).
Jaspers, J. E. et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 3, 68–81 (2013).
Wang, Y. et al. The BRCA1-Δ11q alternative splice isoform bypasses germline mutations and promotes therapeutic resistance to PARP inhibition and cisplatin. Cancer Res. 76, 2778–2790 (2016).
Pettitt, S. J. et al. Genome-wide and high-density CRISPR-Cas9 screens identify point mutations in PARP1 causing PARP inhibitor resistance. Nat. Commun. 9, 1849 (2018).
Michelena, J. et al. Analysis of PARP inhibitor toxicity by multidimensional fluorescence microscopy reveals mechanisms of sensitivity and resistance. Nat. Commun. 9, 2678 (2018).
Gogola, E. et al. Selective loss of PARG restores PARylation and counteracts PARP inhibitor-mediated synthetic lethality. Cancer Cell 35, 950–952 (2019).
Ceccaldi, R. et al. Homologous-recombination-deficient tumours are dependent on Polθ-mediated repair. Nature 518, 258–262 (2015).
Tsuda, M. et al. ALC1/CHD1L, a chromatin-remodeling enzyme, is required for efficient base excision repair. PLoS ONE 12, e0188320 (2017).
Ooka, M. et al. Chromatin remodeler ALC1 prevents replication-fork collapse by slowing fork progression. PLoS ONE 13, e0192421 (2018).
Ahel, D. et al. Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science 325, 1240–1243 (2009).
Ensminger, M. et al. DNA breaks and chromosomal aberrations arise when replication meets base excision repair. J. Cell Biol. 206, 29–43 (2014).
Caldecott, K. W. Single-strand break repair and genetic disease. Nat. Rev. Genet. 9, 619–631 (2008).
Lehmann, A. R. & Fuchs, R. P. Gaps and forks in DNA replication: rediscovering old models. DNA Repair 5, 1495–1498 (2006).
Gottschalk, A. J. et al. Poly(ADP-ribosyl)ation directs recruitment and activation of an ATP-dependent chromatin remodeler. Proc. Natl Acad. Sci. USA 106, 13770–13774 (2009).
Lehmann, L. C. et al. Mechanistic insights into autoinhibition of the oncogenic chromatin remodeler ALC1. Mol. Cell 68, 847–859 (2017).
Singh, H. R. et al. A Poly-ADP-ribose trigger releases the auto-inhibition of a chromatin remodeling oncogene. Mol. Cell 68, 860–871 (2017).
Hanzlikova, H. et al. The importance of poly(ADP-ribose) polymerase as a sensor of unligated Okazaki fragments during DNA replication. Mol. Cell 71, 319–331 (2018).
Hanzlikova, H., Gittens, W., Krejcikova, K., Zeng, Z. & Caldecott, K. W. Overlapping roles for PARP1 and PARP2 in the recruitment of endogenous XRCC1 and PNKP into oxidized chromatin. Nucleic Acids Res. 45, 2546–2557 (2017).
Chittori, S., Hong, J., Bai, Y. & Subramaniam, S. Structure of the primed state of the ATPase domain of chromatin remodeling factor ISWI bound to the nucleosome. Nucleic Acids Res. 47, 9400–9409 (2019).
Karras, G. I. et al. The macro domain is an ADP-ribose binding module. EMBO J. 24, 1911–1920 (2005).
Sellou, H. et al. The poly(ADP-ribose)-dependent chromatin remodeler Alc1 induces local chromatin relaxation upon DNA damage. Mol. Biol. Cell 27, 3791–3799 (2016).
Smith, R., Sellou, H., Chapuis, C., Huet, S. & Timinszky, G. CHD3 and CHD4 recruitment and chromatin remodeling activity at DNA breaks is promoted by early poly(ADP-ribose)-dependent chromatin relaxation. Nucleic Acids Res. 46, 6087–6098 (2018).
Smith, R. et al. Poly (ADP-ribose)-dependent chromatin unfolding facilitates the association of DNA-binding proteins with DNA at sites of damage. Nucleic Acids Res. 47, 11250–11267 (2019).
Tarsounas, M. & Sung, P. The antitumorigenic roles of BRCA1–BARD1 in DNA repair and replication. Nat. Rev. Mol. Cell Biol. 21, 284–299 (2020).
Wong, R. P., García-Rodríguez, N., Zilio, N., Hanulová, M. & Ulrich, H. D. Processing of DNA polymerase-blocking lesions during genome replication is spatially and temporally segregated from replication forks. Mol. Cell 77, 3–16.e4 (2020).
Cong, K. et al. PARPi synthetic lethality derives from replication-associated single-stranded DNA gaps. Preprint at bioRxiv https://doi.org/10.1101/781989 (2019).
Lopes, M., Foiani, M. & Sogo, J. M. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell 21, 15–27 (2006).
Quinet, A. et al. PRIMPOL-mediated adaptive response suppresses replication fork reversal in BRCA-deficient cells. Mol. Cell 77, 461–474 (2020).
Branzei, D. & Szakal, B. Building up and breaking down: mechanisms controlling recombination during replication. Crit. Rev. Biochem. Mol. Biol. 52, 381–394 (2017).
Marians, K. J. Lesion bypass and the reactivation of stalled replication forks. Annu. Rev. Biochem. 87, 217–238 (2018).
Nagaraju, G. & Scully, R. Minding the gap: the underground functions of BRCA1 and BRCA2 at stalled replication forks. DNA Repair 6, 1018–1031 (2007).
Dungrawala, H. et al. The replication checkpoint prevents two types of fork collapse without regulating replisome stability. Mol. Cell 59, 998–1010 (2015).
Liu, X. et al. ERCC6L2 promotes DNA orientation-specific recombination in mammalian cells. Cell Res. 30, 732–744 (2020).
Zimmermann, M. et al. CRISPR screens identify genomic ribonucleotides as a source of PARP-trapping lesions. Nature 559, 285–289 (2018).
DeWeirdt, P. C. et al. Genetic screens in isogenic mammalian cell lines without single cell cloning. Nat. Commun. 11, 752 (2020).
Gier, R. A. et al. High-performance CRISPR-Cas12a genome editing for combinatorial genetic screening. Nat. Commun. 11, 3455 (2020).
Verma, P. et al. RAD52 and SLX4 act nonepistatically to ensure telomere stability during alternative telomere lengthening. Genes Dev. 33, 221–235 (2019).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Di Veroli, G. Y. et al. Combenefit: an interactive platform for the analysis and visualization of drug combinations. Bioinformatics 32, 2866–2868 (2016).
Gatti, M., Imhof, R., Huang, Q., Baudis, M. & Altmeyer, M. The ubiquitin ligase TRIP12 limits PARP1 trapping and constrains PARP inhibitor efficiency. Cell Rep. 32, 107985 (2020).
Quinet, A., Carvajal-Maldonado, D., Lemacon, D. & Vindigni, A. DNA fiber analysis: mind the gap! Methods Enzymol. 591, 55–82 (2017).
Petrovic, J. et al. Oncogenic notch promotes long-range regulatory interactions within hyperconnected 3D cliques. Mol. Cell 73, 1174–1190 (2019).
Acknowledgements
We thank D. Durocher (University of Toronto, Lunenfeld) for sharing hTERT-RPE1 p53−/− BRCA1−/− cells and controls, J. and R. Connaway (Stowers) for ALC1 plasmids, K. Caldecott (University of Sussex) for sharing the GFP-XRCC1 plasmid and hTERT-RPE1 XRCC1−/− and parental control cells, N. Lakin (University of Oxford) for sharing U-2 OS PARP1−/−, PARP2−/− and PARP1/2−/− cells, N. Johnson (Fox Chase) for SUM149PT BRCA1 reversion mutant cell lines and helpful discussions on mouse xenograft experiments and M. Altmeyer (University of Zurich) for helpful suggestions on PARP1 trapping experiments. This work was supported by NIH grants GM101149 and CA17494 (R.A.G.), who is also supported by funds from the Penn Center for Genome Integrity, the Basser Center for BRCA, a V Foundation Team Convergence Award and a Gray Foundation Team Science Award. P.V. was supported by the Ann and Sol Schreiber Mentored Investigator Award (Ovarian Cancer Research Fund Alliance) and pilot funds from the Ovarian Cancer Translational Center for Excellence (UPenn). J.S. was supported by the Michele and Kevah Konner Award through the Basser Center for BRCA.
Author information
Authors and Affiliations
Contributions
P.V., J.S. and R.A.G. designed the study. P.V. did most of the experiments, with assistance from P.V.D., M.D., Y.S., Y.L. and S.P. Z.C. and E.A. performed the CRISPR screen in SUM149PT and CAPAN-1 cells and the RNA-seq experiments. Y.Z. carried out the ATAC-seq experiment under the guidance of R.B.F. W.L. performed the mouse work. L.P. imaged and analysed PARP1 and PARP2 trapping under the guidance of R.H.M. P.V. and R.A.G. wrote the manuscript with contributions from J.S.
Corresponding authors
Ethics declarations
Competing interests
R.A.G. is a founder and scientific advisory board member of RADD Pharmaceuticals. The other authors declare no competing interests.
Additional information
Peer review information Nature Cell Biology thanks Matthias Altmeyer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 ALC1 loss renders olaparib hypersensitivity and proliferation defects in various BRCA-mutant lines.
a, Protein domains ranked on the basis of the CRISPR score (CS) for ola sensitivity in BRCA1-mutant UWB1.289 cells. b, Immunoblot showing depletion of ALC1 using a sgRNA vector with GFP selection marker. GFP-positive cells were sorted and analyzed. The blot is a representative image of two biologically independent experiments. c, GFP competition assay to examine the effects of ALC1 depletion on ola sensitivity in CAPAN-1, SUM149PT and UWB1.289 cells. Ola sensitivity in CAPAN-1 and SUM149PT was confirmed using six and seven independent guides respectively and data for each guide are from one experiment performed at three different ola concentrations. Ola sensitivity in UWB1.289 was confirmed using seven independent guides and data for each guide is mean of two biologically independent experiments in the absence and presence of 50 nM ola concentration. Source data are provided.
Extended Data Fig. 2 ALC1 loss enhances the therapeutic window of olaparib sensitivity in BRCA-mutant cells.
a, Sensitivities of the indicated cell lines to ola using CellTiter-Glo. Data are mean of 2 (hTERT-RPE1) or mean ± s.e.m. of 3 (UWB1.289, SUM149PT and DLD1) biologically independent experiments. b, Representative images (left) and quantification (right) of the clonogenic survival of ALC1-depleted DLD1 WT and BRCA2-mutant cells grown in the presence of increasing concentrations of ola. Data are mean ± s.e.m. from 3 biologically independent experiments. Source data are provided.
Extended Data Fig. 3 Extended analysis of PARPi sensitivity upon ALC1 loss.
a, Sensitivities of the indicated DLD1 BRCA2-/- cells to vel (veliparib), ola and tal in CellTiter-Glo assay. Data are mean ± s.e.m. from n = 3 biologically independent experiments. b, Immunoblot showing ALC1 levels in cells used for xenograft studies (first four left lanes) and in tumors that reached >10.5 mm in any dimension, which is when the mice were euthanized. Data from two biologically independent tumors per condition are shown. c, GFP competition experiment in UWB1.289+BRCA1 addback line to examine the effects of the combined loss of ALC1 and the indicated DNA repair proteins on cell proliferation and ola sensitivity. Data are normalized to Tinitial and indicate mean ± s.e.m. After every two population doublings, cells were passaged (P) and GFP percent was recorded (n=4 independent transductions except for sgFEN1, where n = 6 independent transductions were performed). Source data are provided.
Extended Data Fig. 4 Genomic lesions in PARPi treated ALC1-deficient cells are repaired by SSBR and NHEJ.
a-b, Immunoblot showing levels of ALC1 and XRCC1 in indicated DLD1 (a) and hTERT-RPE1 (b) cells. The western samples were analyzed once to check the efficiency of the sgRNAs for protein depletion before drug sensitivity assays. c, Sensitivities of the indicated DLD1 cells lines to ola and tal using the CellTiter-Glo assay. Data are mean ± s.e.m. from n = 3 biologically independent experiments. d, Sensitivities of the indicated hTERT-RPE1 cells lines to tal using the CellTiter-Glo assay. Data are mean from n=2 biologically independent experiments. e, Sensitivities of the indicated UWB1.289 cell lines to ola using the CellTiter-Glo assay. Data are mean ± s.e.m. from n = 3 biologically independent experiments. f, Quantification of γH2AX-Rad51 foci in indicated cell lines. Cells were fixed 16 hrs. after treatment with 10 Gy ionizing radiation (IR). Median is indicated. p-value determined by Mann–Whitney was derived from n≥54 cells examined over two biologically independent experiments. g, Quantification of γH2AX-Rad51 foci in indicated cell lines. Cells were treated with 5 µM ola for 24 hrs. before fixation. Median is indicated. p-value determined by Mann–Whitney was derived from n≥114 cells examined over three biologically independent experiments. h, Representative images and quantification of radials (indicated by red arrowheads) and breaks (indicated by yellow arrowheads) in the indicated UWB1.289 cell lines, post treatment with 1 µM ola for 24 hrs. For each experiment, at least 50 spreads were analyzed per sample. Data are mean from two biologically independent experiments. Source data are provided.
Extended Data Fig. 5 ALC1 deficiency results in increased trapping of PARP1 and PARP2 by PARPi upon DNA damage.
a-b Representative images (left) and quantification (right) of PARP1(a) and PARP2 (b) trapping in UWB1.289 cells. Indicated treatments were performed for 4 hours. Median is indicated. p-value determined by Mann–Whitney was derived from n≥107 cells sampled over two biologically independent experiments.
Extended Data Fig. 6 ALC1 loss confers MMS sensitivity and results in replication-coupled gaps.
a, Representative images of Rad51-γH2AX foci in U-2 OS (left) and UWB1.289+BRCA1 (right) cell lines Scale bar: 10 microns. Images represent n≥67 cells examined over two biologically independent experiments. b, Representative images of γH2AX signal in EdU negative (left) and positive (right) hTERT-RPE1 BRCA1-/- cells. Scale bar: 10 microns. Images represent n≥99 cells examined over two biologically independent experiments. c, Sensitivities of the indicated cells lines to MMS and CPT using the CellTiter-Glo assay. Data are mean ± s.e.m. from n=3 biologically independent experiments. d, Sensitivities of the indicated hTERT-RPE1 cells lines to MMS and CPT using the CellTiter-Glo assay. Data are mean ± s.e.m. from n = three biologically independent experiments. e, Representative images of fibers from the S1 nuclease experiment. Scale bar: 2 microns. Images represent n≥75 fibers examined over two biologically independent experiments. Source data are provided.
Extended Data Fig. 7 ALC1 is recruited to the damaged chromatin under conditions of reduced PARylation.
a, Schematic of the experiment. b-c, Representative images (b) and quantification (c) of HA-ALC1 localization to chromatin upon indicated treatments in U-2 OS cells. d, Schematic of the experiment. e-f, Representative images (e) and quantification (f) of HA-ALC1 localization to chromatin upon indicated treatments in SUM149PT cells. Scale bar, 10 microns. The median value was normalized to untreated control. Data are mean ± s.e.m. from n = three biologically independent experiments, p-value, unpaired Student’s t-test. For each experiment, at least 50 cells were analyzed per sample. Source data are provided.
Extended Data Fig. 8 ATPase activity, H4 interaction and macrodomains of ALC1 are essential for protecting BRCA-mutant cells from ola hypersensitivity.
a, Representative images (left) and quantification (right) of the clonogenic survival assay using hTERT-RPE1 BRCA1-/- cells expressing sgALC1 and the indicated ALC1 mutants treated with ola (1 nM). Data are mean from two biologically independent experiments. b, Representative images (left) and quantification (right) of the clonogenic survival assay (left) using SUM149PT cells expressing sgALC1 and ALC1 K77R mutant treated with ola (0.5 nM). Data are mean ± s.e.m from n = three independent experiments. Number of colonies in the ola treated condition were normalized to its respective untreated counterpart. c, Sequence alignment of various chromatin remodelers using Clustal Omega. Histone H4 interacting residues as predicted by PDB:6PWF are highlighted and marked by a blue star. d, Immunoblots showing interactions of FLAG ALC1 WT and FLAG ALC1 D377A+ D381A with histone H4. Experiment was repeated twice with similar outcomes. e, Representative images of the clonogenic survival assay (left) and quantification (right) of SUM149PT cells expressing sgALC1 and indicated ALC1 macrodomain mutants treated with ola (1 nM). Data are mean ± s.e.m. from n = three independent experiments. Number of colonies in the ola treated condition were normalized to its respective untreated counterpart. Source data are provided.
Extended Data Fig. 9 ALC1 co-operates with PARP activity to permit association of repair proteins with chromatin.
a, DLD1 BRCA2–/– cells were fractionated and the chromatin-bound proteins were immunoblotted. Cells were treated with 5 µM ola for 4 hrs. Data for DLD1 BRCA2–/– cells are from the same sample, from two different western blots and the histone levels for each blot are shown by the ponceau staining. The data for XRCC1 is representative of 5 biologically independent experiments and the data for NTHL1 and APE1 is representative of 3 biologically independent experiments. b, Immunoblot of whole cell lysates of DLD1 BRCA2-/- cells showing total proteins levels upon ALC1 depletion and PARPi treatment. Cells were treated with indicated PARPi for 4 hrs. Data is representative of two biologically independent experiments. c, UWB1.289 cells were fractionated and the chromatin-bound proteins were immunoblotted. Cells were treated with 1 µM tal for 4 hrs. Data for XRCC1 is representative of 4 biologically independent experiments and the data for APE1 is representative of 3 biologically independent experiments. d, Immunoblot showing expression levels of HA-XRCC1. The blot was performed once to access the expression level of the tagged protein. e, Schematic of the IF experiment. f-g, Representative images (f) and quantification (g) of HA-XRCC1 localization to chromatin upon indicated treatments. Scale bar, 50 microns. Data are mean ± s.e.m. from n = three biologically independent experiments, p-value, unpaired Student’s t-test. For each cell line, the median value upon MMS treatment was normalized to its respective untreated control. Source data are provided.
Extended Data Fig. 10 ALC1 loss synergistically enhances IR sensitivity at low olaparib doses.
a-b Representative images of two independent clonogenic survival assay to monitor the effect of combining low doses of ola and IR upon ALC1 depletion in UWB1.289 (a) and hTERT-RPE1 BRCA1-/- cells (b). c-d, Quantification of clonogenic survival (c) and heat map of bliss scores (d) obtained from BRCA1–/– hTERT-RPE1 cells treated with the indicated doses of ola and IR. Data are mean from two biologically independent experiments. Bliss score >0, synergistic; Bliss score <0, antagonistic; Bliss score = 0, additive. Number of colonies in IR-treated conditions were normalized to their respective un-irradiated counterparts. Colonies with more than 50 cells were included in the analysis. Source data are provided.
Supplementary information
Supplementary Table 1
Differential CRISPR scores.
Supplementary Table 2
ATAC-seq data.
Supplementary Table 3
RNA-seq data.
Supplementary Table 4
List of sgRNAs and primers.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blot.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blot.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blot.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blot.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blot.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blot.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blot.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blot.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Verma, P., Zhou, Y., Cao, Z. et al. ALC1 links chromatin accessibility to PARP inhibitor response in homologous recombination-deficient cells. Nat Cell Biol 23, 160–171 (2021). https://doi.org/10.1038/s41556-020-00624-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-020-00624-3
This article is cited by
-
TRIM33 loss in multiple myeloma is associated with genomic instability and sensitivity to PARP inhibitors
Scientific Reports (2024)
-
Transcription–replication conflicts underlie sensitivity to PARP inhibitors
Nature (2024)
-
Asymmetric nucleosome PARylation at DNA breaks mediates directional nucleosome sliding by ALC1
Nature Communications (2024)
-
Targeting DNA damage response pathways in cancer
Nature Reviews Cancer (2023)
-
Break-induced replication orchestrates resection-dependent template switching
Nature (2023)