Abstract
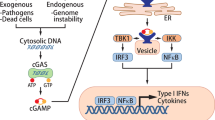
Sensing cytosolic DNA through the cGAS–STING pathway constitutes a widespread innate immune mechanism to monitor cellular damage and microbial invasion. Evading this surveillance is crucial in tumorigenesis, but the process remains largely unexplored. Here, we show that the receptor tyrosine kinase HER2 (also known as ErbB-2 or Neu) potently inhibits cGAS–STING signalling and prevents cancer cells from producing cytokines, entering senescence and undergoing apoptosis. HER2, but not EGFR, associates strongly with STING and recruits AKT1 (also known as PKB) to directly phosphorylate TBK1, which prevents the TBK1–STING association and TBK1 K63-linked ubiquitination, thus attenuating STING signalling. Unexpectedly, we observed that DNA sensing robustly activates the HER2–AKT1 axis, resulting in negative feedback. Accordingly, genetic or pharmacological targeting of the HER2–AKT1 cascade augments damage-induced cellular senescence and apoptosis, and enhances STING-mediated antiviral and antitumour immunity. Thus, our findings reveal a critical function of the oncogenic pathway in innate immune regulation and unexpectedly connect HER2–AKT1 signalling to the surveillance of cellular damage and antitumour immunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in this paper and from the corresponding author on reasonable request. The statistical source data for Figs. 1–7 and Supplementary Figs. 1–7 are provided in Supplementary Table 1, and the source data for Fig. 4e and Supplementary Fig. 4b, that is, the mass spectrometry analysis of the TBK1 modification, is provided in Supplementary Table 5. Mass spectrometry data have been deposited in ProteomeXchange Consortium with the primary accession code PXD013957 via the iProX partner repository.
References
Gao, P. et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 153, 1094–1107 (2013).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Chen, Q., Sun, L. & Chen, Z. J. Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat. Immunol. 17, 1142–1149 (2016).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Roers, A., Hiller, B. & Hornung, V. Recognition of endogenous nucleic acids by the innate immune system. Immunity 44, 739–754 (2016).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Zhong, B. et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29, 538–550 (2008).
Sun, W. et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc. Natl Acad. Sci. USA 106, 8653–8658 (2009).
Moretti, J. et al. STING senses microbial viability to orchestrate stress-mediated autophagy of the endoplasmic reticulum. Cell 171, 809–823.e813 (2017).
Luo, W. W. et al. iRhom2 is essential for innate immunity to DNA viruses by mediating trafficking and stability of the adaptor STING. Nat. Immunol. 17, 1057–1066 (2016).
Saitoh, T. et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc. Natl Acad. Sci. USA 106, 20842–20846 (2009).
Dobbs, N. et al. STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe 18, 157–168 (2015).
Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 (2003).
Fitzgerald, K. A. et al. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 (2003).
Gao, D. et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl Acad. Sci. USA 112, E5699–E5705 (2015).
Crampton, S. P. & Bolland, S. Spontaneous activation of RNA-sensing pathways in autoimmune disease. Curr. Opin. Immunol. 25, 712–719 (2013).
Dou, Z. et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550, 402–406 (2017).
Yang, H., Wang, H., Ren, J., Chen, Q. & Chen, Z. J. cGAS is essential for cellular senescence. Proc. Natl Acad. Sci. USA 114, E4612–E4620 (2017).
Gluck, S. et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 19, 1061–1070 (2017).
Fu, J. et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 7, 283ra252 (2015).
Demaria, O. et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl Acad. Sci. USA 112, 15408–15413 (2015).
Deng, L. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014).
Bakhoum, S. F. et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472 (2018).
Mackenzie, K. J. et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548, 461–465 (2017).
Tang, C. H. et al. Agonist-mediated activation of STING induces apoptosis in malignant B cells. Cancer Res. 76, 2137–2152 (2016).
Corrales, L. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 11, 1018–1030 (2015).
Liu, S. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630 (2015).
Zhang, C. et al. Structural basis of STING binding with and phosphorylation by TBK1. Nature 567, 394–398 (2019).
Wild, P. et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333, 228–233 (2011).
Hou, F. et al. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461 (2011).
Porritt, R. A. & Hertzog, P. J. Dynamic control of type I IFN signalling by an integrated network of negative regulators. Trends Immunol. 36, 150–160 (2015).
Liu, J., Qian, C. & Cao, X. Post-translational modification control of innate immunity. Immunity 45, 15–30 (2016).
Zhang, Q. et al. Hippo signalling governs cytosolic nucleic acid sensing through YAP/TAZ-mediated TBK1 blockade. Nat. Cell Biol. 19, 362–374 (2017).
Xiang, W. et al. PPM1A silences cytosolic RNA sensing and antiviral defense through direct dephosphorylation of MAVS and TBK1. Sci. Adv. 2, e1501889 (2016).
Liu, S. et al. Lck/Hck/Fgr-Mediated Tyrosine Phosphorylation Negatively Regulates TBK1 to Restrain Innate Antiviral Responses. Cell Host Microbe 21, 754–768.e755 (2017).
Moasser, M. M. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 26, 6469–6487 (2007).
Arteaga, C. L. & Engelman, J. A. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 25, 282–303 (2014).
Gennari, R. et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin. Cancer Res. 10, 5650–5655 (2004).
Kroemer, G., Senovilla, L., Galluzzi, L., Andre, F. & Zitvogel, L. Natural and therapy-induced immunosurveillance in breast cancer. Nat. Med. 21, 1128–1138 (2015).
Sistigu, A. et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 20, 1301–1309 (2014).
Park, S. et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 18, 160–170 (2010).
Stagg, J. et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl Acad. Sci. USA 108, 7142–7147 (2011).
Fang, R. et al. MAVS activates TBK1 and IKKepsilon through TRAFs in NEMO dependent and independent manner. PLoS Pathog. 13, e1006720 (2017).
Liu, S. et al. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. eLife 2, e00785 (2013).
Song, G. et al. E3 ubiquitin ligase RNF128 promotes innate antiviral immunity through K63-linked ubiquitination of TBK1. Nat. Immunol. 17, 1342–1351 (2016).
Tu, D. et al. Structure and ubiquitination-dependent activation of TANK-binding kinase 1. Cell Rep. 3, 747–758 (2013).
Shu, C. et al. Structural insights into the functions of TBK1 in innate antimicrobial immunity. Structure 21, 1137–1148 (2013).
Larabi, A. et al. Crystal structure and mechanism of activation of TANK-binding kinase 1. Cell Rep. 3, 734–746 (2013).
Li, S., Wang, L., Berman, M., Kong, Y. Y. & Dorf, M. E. Mapping a dynamic innate immunity protein interaction network regulating type I interferon production. Immunity 35, 426–440 (2011).
Jaishankar, D., et al. An off-target effect of BX795 blocks herpes simplex virus type 1 infection of the eye. Sci. Transl. Med. 10, eaan5861 (2018).
Kishi, S. et al. The identification of zebrafish mutants showing alterations in senescence-associated biomarkers. PLoS Genet. 4, e1000152 (2008).
Gulen, M. F. et al. Signalling strength determines proapoptotic functions of STING. Nat. Commun. 8, 427 (2017).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
Melki, I. et al. Disease-associated mutations identify a novel region in human STING necessary for the control of type I interferon signaling. J. Allergy Clin. Immunol. 140, 543–552 (2017).
Ueki, I. F. et al. Respiratory virus-induced EGFR activation suppresses IRF1-dependent interferon λ and antiviral defense in airway epithelium. J. Exp. Med. 210, 1929–1936 (2013).
Cho, H. S. et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421, 756–760 (2003).
Ahmad, I. et al. HER2 overcomes PTEN (loss)-induced senescence to cause aggressive prostate cancer. Proc. Natl Acad. Sci. USA 108, 16392–16397 (2011).
Li, T. & Chen, Z. J. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 215, 1287–1299 (2018).
Zhao, P. et al. TBK1 at the crossroads of inflammation and energy homeostasis in adipose tissue. Cell 172, 731–743 (2018).
Pilli, M. et al. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity 37, 223–234 (2012).
Gui, X. et al. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 567, 262–266 (2019).
Ou, Y. H. et al. TBK1 directly engages Akt/PKB survival signaling to support oncogenic transformation. Mol. Cell 41, 458–470 (2011).
Xie, X. et al. IκB kinase ε and TANK-binding kinase 1 activate AKT by direct phosphorylation. Proc. Natl Acad. Sci. USA 108, 6474–6479 (2011).
Li, X. D. et al. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341, 1390–1394 (2013).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Seo, G. J. et al. Akt kinase-mediated checkpoint of cGAS DNA sensing pathway. Cell Rep. 13, 440–449 (2015).
Cho, H. et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292, 1728–1731 (2001).
Chen, W. S. et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the akt1 gene. Genes Dev. 15, 2203–2208 (2001).
Xu, P. et al. Innate antiviral host defense attenuates TGF-β function through IRF3-mediated suppression of Smad signaling. Mol. Cell 56, 723–737 (2014).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Acknowledgements
We are grateful to J. Han and Z. Jiang for the HSV-1 and VacV viruses, X. Wang and X. Guo for reagents, and Y. J. Zhang and B. Yang for their helpful discussions. This research was sponsored by the National Science Foundation for Distinguished Young Scholars (grant no. 31725017 to P.X.), NSFC Projects (grant nos 31830052 and 81472665 to P.X.), MoST 973 Plan (grant no. 2015CB553800 to P.X.), Initiative Postdocs Supporting Program (Q.Z.), Laboratory of Animal Virology of MoA (Q.Z.), and Project 985 and the Fundamental Research Funds for the Central Universities to the Life Sciences Institute at Zhejiang University. P.X. is a scholar in the National 1000 Young Talents Program.
Author information
Authors and Affiliations
Contributions
S.W., Q.Z. and F.Z. carried out most of the experiments. F.M., S.L., R.Z. and Q.W. contributed to several experiments. X.L., L.S., J.H., J.Q., Z.X., S.O., H.S., X.-H.F. and J.Z. helped with the data analyses and were involved in discussions of the data. P.X. conceived the study and experimental design and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 HER2 suppresses cGAS-STING signaling to dampen cytosolic DNA sensing.
(S1A), TBK1 activation, as indicated by the IRF3-responsive ISRE reporter (left panel) or S172 phosphorylation (right panel), was profoundly inhibited upon coexpression with HER2 but not with other RTKs from the ERBB family. Protein expression and kinase activities were verified by immunoblotting. (S1B and S1C), Activating mutant of EGFR (L858R) potentiated TBK1 activation, regardless expression levels of HER2. (S1D), HER2 inhibited STING signaling in a dose-dependent manner, as revealed by the IRF3-responsive IFNβ reporter. (S1E), HER2 was unable to inhibit the transcriptional activity of activated IRF3 (5SD). n=4 independent experiments (mean±SEM). (S1F), The ICD of HER2 suppressed STING signaling even more strongly than did full-length HER2, as revealed by the IRF3-responsive ISRE reporter (left panel) or by immunoblotting for phospho-TBK1 S172 (right panel). (S1G), Lapatinib treatment enhanced STING signaling as revealed by the IRF3-responsive IFNβ reporter assay. (S1H), Inhibition of HER2 by lapatinib or ARRY-380 in NMuMG cells enhanced cGAMP-induced activation of endogenous TBK1. Ratio of pTBK1/total TBK1 was determined and indicated. (S1I), cGAMP-stimulated activation of endogenous TBK1 was substantially boosted by lapatinib treatment in HER2-driven tumor lines BT474. (S1J), Cytosolic DNA sensing stimulated by cytosolic exposure of poly(dA:dT) was attenuated by HER2 expression; this loss was recovered upon treatment with the HER2 inhibitor lapatinib. (S1K and S1L), Enhanced cytosolic DNA sensing in HER2-knockout cells but suppressed in HER2-rescue cells was detected by the activation of endogenous TBK1 and IRF3, which was stimulated either with cGAMP (S1K) or with TpdAdT (S1L). Activation of EGFR signaling by ligand EGF enhanced cytosolic DNA sensing, while activation of HER3/HER4 signaling by ligand NRG1-β1 did not alter this signaling, regardless the presence or absence of HER2. (S1M), HER2-knockout NMuMG cells (left panel) and HCT116 cells (right panel) were generated by CRISPR-mediated genome editing and verified by sequential immunoprecipitation and immunoblotting of endogenous HER2. (S1N), siRNA-mediated HER2 depletion in BT474 cells enhanced cGAMP-stimulated activation of endogenous TBK1. Unless specified, n=3 independent experiments (mean±SEM). P values are indicated, by ANOVA test and Bonferroni correction in statistics. Unprocessed images of blots are shown in Supplementary Figure 8. Statistics source data are provided in Supplementary Table 1.
Supplementary Figure 2 HER2 associates with STING and disrupts the assembly of the STING signalosome.
(S2A), STING aggregation better overlapped with the Golgi marker in response to HER2 inhibition at 3 hours post-cGAMP administration. Scale bars=20 μm. (S2B), cGAMP-stimulated aggregation of endogenous STING in HER2-driven tumor line BT474 was comparatively mild but was obviously improved by lapatinib treatment. (S2C), A strong association between STING and HER2, but not between STING and EGFR, was detected in an immunoprecipitation assay with proteins that were cotransfected. (S2D), Association between STING and EGFR was not upregulated in the presence of cotransfected HER2, indicating inefficiency of STING to interact with EGFR-HER2 heterodimers. (S2E and S2F), Immunofluorescence under confocal microscopy revealed an obvious colocalization of inducible HER2 and endogenous STING in HER2 Tet-On DLD1 cells, within puncta of STING formed by cGAMP stimulation (arrowed) (S2E). Upon cGAMP stimulation, HER2 was apparently translocated from dispersive distribution along plasma membrane and ER to forming puncta at ER (arrowed) (S2F). Lapatinib affected the cellular localization of HER2, which showed a reduced distribution to the ER compartments (S2F). Scale bars=20 μm. (S2G and S2H), An increased colocalization of endogenous or inducible HER2 in Golgi marker (arrowed) was observed upon extended treatment of cGAMP in HER2-driven tumor line BT474 (S2G) or HER2 Tet-On DLD1 cells (S2H). Scale bars=20 µm. (S2I), Coimmunoprecipitation assays of HER2 and STING truncations showed that the intact C-terminal domain (CTD) (a.a. 139-379) of STING and the intact ICD of HER2 were necessary and sufficient for their interaction. (S2J), K63-linked ubiquitination, an indicator of TRAF6 activation, was impeded by HER2. Unless specified, n=3 independent experiments. Unprocessed images of blots are shown in Supplementary Figure 8.
Supplementary Figure 3 The HER2-AKT1 axis is activated and required for HER2-mediated suppression of cytosolic DNA sensing.
(S3A and S3B), No HER2-mediated TBK1 tyrosine phosphorylation was observed (S3A), where LCK-mediated tyrosine phosphorylation of TBK1 was set as a positive control. Similarly, no clear signal of HER2-mediated tyrosine phosphorylation on STING or TRAF3/6 was detected (S3B), where tyrosine autophosphorylation of HER2 served as the positive control. (S3C), Cytosolic sensing of the DNA analog poly(dA:dT) induced AKT1 activation in mouse PMs, which was stopped by the HER2 inhibitor lapatinib. (S3D), Cytosolic sensing of DNA analogs also induced the activation of HER2, along with the phosphorylation of AKT1, in HER2 Tet-On DLD1 cells. (S3E), A potent and synergistic augmentation of STING signaling was observed in HEK293 cells when both HER2 and AKT1 were blocked by their inhibitors. (S3F), Activation of ectopically expressed TBK1 (Myc-tagged) was stronger in AKT1-knockout HEK293 cells, which cannot be repressed by HER2 in these cells. (S3G and S3H), Deletion of both AKT2 and AKT3 by CRISPR-based strategy did not affect the HER2-induced suppression or EGFR-induced potentiation of TBK1 activation. (S3I), Enhanced STING signaling was observed when wild-type or L858R EGFR was cotransfected; this enhancement was independent of AKT1. (S3J), EGFR enhanced the interaction between TBK1 and IRF3, which was unaffected by AKT inhibitor MK2206. Unless specified, n=3 independent experiments (mean±SEM). P values are indicated, by ANOVA test and Bonferroni correction in statistics. Unprocessed images of blots are shown in Supplementary Figure 8. Statistics source data are provided in Supplementary Table 1.
Supplementary Figure 4 AKT1 phosphorylates TBK1 at S510 to impede the STING signalosome.
(S4A), The AKT1-mediated modification on key components of the MAVS signalosome was examined by immunoblotting of an antibody recognizing the phospho-AKT substrate (phospho-RXXS*/T*), the results of which showed that TBK1, but not RIG-I, MAVS, or IRF3, was robustly phosphorylated by AKT1. (S4B), The PSMs of TBK1 mass spectrometry revealed that TBK1 was robustly modified by AKT1 at S510 residue, which was well blocked by treatment of the AKT inhibitor MK2206. (S4C), Mutating R507 to alanine (R507A) to disrupt the classical AKT substrate motif rendered TBK1 unrecognized by the phospho-AKT substrate antibody, whereas mutating L505 to arginine (L505R) that mimicked the perfect AKT substrate motif resulted in a stronger phosphorylation signal on TBK1 by endogenous or transfected AKT1. (S4D), Mutating TBK1 S499 into aspartate (S499D) enhanced AKT1-mediated TBK1 phosphorylation. In contrast, mutating TBK1 proximal residues in PRM including T514, T517, and S518 residues into alanines (3TS/A), which reversed the constitutive state of TBK1 phosphorylation, impeded the AKT1-mediated TBK1 phosphorylation. (S4E), The TBK1-IRF3 interaction was attenuated when TBK1 was mutated to simulate the constitutive AKT1-induced phosphorylation. (S4F), Cotransfection of AKT1, but not AKT2 or AKT3, resulted in the effective TBK1 modification. (S4G), Two clones of DLD1 knock-in cells with point mutagenesis of TBK1 S510A were generated by CRISPR-mediated genome editing, and verified by PCR amplification of genomic DNA and subsequent sequencing. Unless specified, n=3 independent experiments. Unprocessed images of blots are shown in Supplementary Figure 8.
Supplementary Figure 5 HER2 inhibits antiviral defense initiated by cytosolic DNA sensing.
(S5A), Upon genetic ablation of STING in DLD1 cells by CRISPR-based genome editing, the effect of HER2 on potentiating HSV-1 infection was abolished, as revealed by the FACS of viral replication (GFP+) cells. (S5B), An arbitrary standard of ocular disease scoring (0 to 5, 5 being severe) of HSV-1 corneal infection model was indicated, blindly determined by two observers. Unless specified, n=3 independent experiments.
Supplementary Figure 6 HER2 prevents damage-induced cellular senescence and apoptosis.
(S6A), DNA damage-induced TBK1 activation was impeded in STING-knockout DLD1 cells. (S6B and S6C), The effect of HER2 on alleviating HU-induced cellular senescence was entirely dependent of the presence of STING, as revealed by β-Gal staining (S6B) and the SASP (S6C). Statistics of β-Gal staining cells were calculated. Scale bars=100 μm. (S6D-S6F), Coimmunoprecipitation assay revealed that zebrafish STING (zSTING) interacted with full-length HER2 or its ICD (S6D). Zebrafish TBK1 (zTBK1) delivered signal to C-terminus phosphorylation of human IRF3 (S6E) and IRF3 transactivation (S6F), which was suppressed by human HER2 (S6F). (S6G and S6H), HER2 induction prevented the DNA damage-induced apoptosis of DLD1 cells, as revealed by microscopy (left panels, S6G) or FACS (right panels, S6G) of apoptotic cells. This effect of HER2 was completely dependent of the presence of STING (S6H). Scale bars=100 μm. Unless specified, n=3 independent experiments (mean±SEM). P values are indicated, by ANOVA test and Bonferroni correction in statistics. Unprocessed images of blots are shown in Supplementary Figure 8. Statistics source data are provided in Supplementary Table 1.
Supplementary Figure 7 HER2 protects cancer cells from STING-mediated antitumor immunity.
(S7A), Either expression of the STING SAVI mutant (R281Q) or cellular damage induced by CPT or HU resulted in cellular senescence of B16-F10 melanoma cells, as measured by the SASP cytokine MMP12; this response was abrogated by HER2 expression. (S7B and S7C), Stable expression of STING SAVI mutant and HER2 in B16-F10 melanoma cells were indicated (S7B). The ectopic expression of human HER2 delivered downstream signals in B16 cells, as revealed by the activation of AKT and ERK MAPK signaling (S7C). (S7D), Tumor volume of murine xenograft growth of B16-F10 expressing STING SAVI mutant and HER2 was measured at 16 dpti, in the same experiment of Fig. 7c. (S7E), Hematoxylin and eosin (HE) staining of melanoma tissues revealed an increase in the number of immune cells (circled) in tumors harboring active STING; this increase was partially reversed by HER2 expression. Scale bars=20 μm. (S7F), The robust increase in infiltrating CD4+ and CD8+ T cells in melanoma harboring STING SAVI mutant was attenuated by administration of the neutralizing antibody against IFNAR1, or by ectopic expression of HER2 (upper panel). Scale bars=20 μm. Statistics of IHC-positive cells were calculated (lower panel). Unless specified, n=3 independent experiments (mean±SEM). P values are indicated, by ANOVA test and Bonferroni correction in statistics. Unprocessed images of blots are shown in Supplementary Figure 8. Statistics source data are provided in Supplementary Table 1.
Supplementary information
Supplementary Information
Supplementary Figures 1–8, Supplementary Table titles/legends
Supplementary Table 1
Statistics Source Data
Supplementary Table 2
Plasmid Information
Supplementary Table 3
Antibody Information
Supplementary Table 4
Oligo Information
Supplementary Table 5
MS Results of TBK1 Modification
Rights and permissions
About this article
Cite this article
Wu, S., Zhang, Q., Zhang, F. et al. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nat Cell Biol 21, 1027–1040 (2019). https://doi.org/10.1038/s41556-019-0352-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-019-0352-z
This article is cited by
-
Hypoxic glioblastoma-cell-derived extracellular vesicles impair cGAS-STING activity in macrophages
Cell Communication and Signaling (2024)
-
A landscape of gene expression regulation for synovium in arthritis
Nature Communications (2024)
-
Harnessing innate immune pathways for therapeutic advancement in cancer
Signal Transduction and Targeted Therapy (2024)
-
Innate immune sensing of lysosomal dysfunction drives multiple lysosomal storage disorders
Nature Cell Biology (2024)
-
PTK2B promotes TBK1 and STING oligomerization and enhances the STING-TBK1 signaling
Nature Communications (2023)