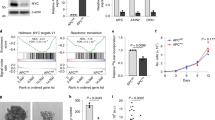
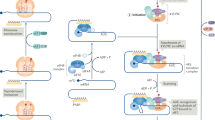
Abstract
The c-Myc oncogene drives malignant progression and induces robust anabolic and proliferative programmes leading to intrinsic stress. The mechanisms enabling adaptation to MYC-induced stress are not fully understood. Here we reveal an essential role for activating transcription factor 4 (ATF4) in survival following MYC activation. MYC upregulates ATF4 by activating general control nonderepressible 2 (GCN2) kinase through uncharged transfer RNAs. Subsequently, ATF4 co-occupies promoter regions of over 30 MYC-target genes, primarily those regulating amino acid and protein synthesis, including eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), a negative regulator of translation. 4E-BP1 relieves MYC-induced proteotoxic stress and is essential to balance protein synthesis. 4E-BP1 activity is negatively regulated by mammalian target of rapamycin complex 1 (mTORC1)-dependent phosphorylation and inhibition of mTORC1 signalling rescues ATF4-deficient cells from MYC-induced endoplasmic reticulum stress. Acute deletion of ATF4 significantly delays MYC-driven tumour progression and increases survival in mouse models. Our results establish ATF4 as a cellular rheostat of MYC activity, which ensures that enhanced translation rates are compatible with survival and tumour progression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
tRNA microarray and ChIP–seq data that support the findings of this study have been deposited in the Gene Expression Omnibus under accession codes GSE116812 and GSE117240, respectively.
The human COAD, BRCA and SARC datasets were derived from the TCGA Data Hub on the University of California Santa Cruz Xena platform (http://xena.ucsc.edu/). The dataset derived from this resource that supports the findings of this study is available in the following links. COAD: https://xenabrowser.net/datapages/?dataset=TCGA.COAD.sampleMap%2FHiSeqV2_PANCAN&host=https%3A%2F%2Ftcga.xenahubs.net&removeHub=https%3A%2F%2Flocal.xena.ucsc.edu%3A7223; BRCA: https://xenabrowser.net/datapages/?dataset=TCGA.BRCA.sampleMap%2FHiSeqV2_PANCAN&host=https%3A%2F%2Ftcga.xenahubs.net&removeHub=https%3A%2F%2Flocal.xena.ucsc.edu%3A7223; SARC: https://xenabrowser.net/datapages/?dataset=TCGA.SARC.sampleMap%2FHiSeqV2_PANCAN&host=https%3A%2F%2Ftcga.xenahubs.net&removeHub=https%3A%2F%2Flocal.xena.ucsc.edu%3A7223. The human DLBCL data were derived from the National Cancer Institute Center for Cancer Genomics website: https://gdc.cancer.gov. The dataset derived from this resource that supports the findings of this study is available at https://gdc.cancer.gov/about-data/publications/DLBCL-2018.
Statistics source data for graphical representations and statistical analyses in Figs. 1–6 and Supplementary Figs. 1–6 are provided in Supplementary Table 3. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Change history
17 July 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Pakos-Zebrucka, K. et al. The integrated stress response. EMBO Rep. 17, 1374–1395 (2016).
Wek, R. C., Jiang, H. Y. & Anthony, T. G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 34, 7–11 (2006).
Dong, J., Qiu, H., Garcia-Barrio, M., Anderson, J. & Hinnebusch, A. G. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell 6, 269–279 (2000).
Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Dey, S. et al. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J. Clin. Invest. 125, 2592–2608 (2015).
Gardner, B. M., Pincus, D., Gotthardt, K., Gallagher, C. M. & Walter, P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 5, a013169 (2013).
Ye, J. et al. The GCN2–ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 29, 2082–2096 (2010).
Bi, M. et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 24, 3470–3481 (2005).
Fels, D. R. & Koumenis, C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol. Ther. 5, 723–728 (2006).
Ozcan, U. et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell 29, 541–551 (2008).
Hart, L. S. et al. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J. Clin. Invest. 122, 4621–4634 (2012).
Tameire, F., Verginadis, I. I. & Koumenis, C. Cell intrinsic and extrinsic activators of the unfolded protein response in cancer: mechanisms and targets for therapy. Semin. Cancer Biol. 33, 3–15 (2015).
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010).
Iritani, B. M. & Eisenman, R. N. c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc. Natl Acad. Sci. USA 96, 13180–13185 (1999).
Stine, Z. E., Walton, Z. E., Altman, B. J., Hsieh, A. L. & Dang, C. V. MYC, metabolism, and cancer. Cancer Discov. 5, 1024–1039 (2015).
Barna, M. et al. Suppression of Myc oncogenic activity by ribosomal protein haploinsufficiency. Nature 456, 971–975 (2008).
Lin, C. J. et al. Targeting synthetic lethal interactions between Myc and the eIF4F complex impedes tumorigenesis. Cell Rep. 1, 325–333 (2012).
Harding, H. P., Zhang, Y., Bertolotti, A., Zeng, H. & Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 (2000).
Nagy, P., Varga, A., Pircs, K., Hegedus, K. & Juhasz, G. Myc-driven overgrowth requires unfolded protein response-mediated induction of autophagy and antioxidant responses in Drosophila melanogaster. PLoS Genet. 9, e1003664 (2013).
Wang, Y. et al. Amino acid deprivation promotes tumor angiogenesis through the GCN2/ATF4 pathway. Neoplasia 15, 989–997 (2013).
Sood, R., Porter, A. C., Olsen, D. A., Cavener, D. R. & Wek, R. C. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2ɑ. Genetics 154, 787–801 (2000).
Gomez-Roman, N., Grandori, C., Eisenman, R. N. & White, R. J. Direct activation of RNA polymerase III transcription by c-Myc. Nature 421, 290–294 (2003).
Wu, L. et al. Novel small-molecule inhibitors of RNA polymerase III. Eukaryot. Cell 2, 256–264 (2003).
Avcilar-Kucukgoze, I. et al. Discharging tRNAs: a tug of war between translation and detoxification in Escherichia coli. Nucleic Acids Res. 44, 8324–8334 (2016).
Han, J. et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell. Biol. 15, 481–490 (2013).
Chen, H. et al. ATF4 regulates SREBP1c expression to control fatty acids synthesis in 3T3-L1 adipocytes differentiation. Biochim. Biophys. Acta 1859, 1459–1469 (2016).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976 (2017).
Hay, N. & Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 (2004).
Pause, A. et al. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 371, 762–767 (1994).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006).
Hsieh, A. C. et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP–eIF4E. Cancer Cell 17, 249–261 (2010).
Adams, J. M. et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318, 533–538 (1985).
Anthony, T. G. et al. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J. Biol. Chem. 279, 36553–36561 (2004).
Zhang, P. et al. The GCN2 eIF2ɑ kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell Biol. 22, 6681–6688 (2002).
Masuoka, H. C. & Townes, T. M. Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood 99, 736–745 (2002).
Pytel, D. et al. PERK is a haploinsufficient tumor suppressor: gene dose determines tumor-suppressive versus tumor promoting properties of perk in melanoma. PLoS Genet. 12, e1006518 (2016).
Gao, Y. et al. PERK is required in the adult pancreas and is essential for maintenance of glucose homeostasis. Mol. Cell Biol. 32, 5129–5139 (2012).
Nguyen, H. G. et al. Development of a stress response therapy targeting aggressive prostate cancer. Sci. Transl. Med. 10, eaar2036 (2018).
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007).
Ruggero, D. The role of Myc-induced protein synthesis in cancer. Cancer Res. 69, 8839–8843 (2009).
Xu, C. & Ng, D. T. Glycosylation-directed quality control of protein folding. Nat. Rev. Mol. Cell Biol. 16, 742–752 (2015).
Novoa, I., Zeng, H., Harding, H. P. & Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2ɑ. J. Cell Biol. 153, 1011–1022 (2001).
Tettweiler, G., Miron, M., Jenkins, M., Sonenberg, N. & Lasko, P. F. Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev. 19, 1840–1843 (2005).
Kremer, C. L. et al. Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate 66, 1203–1212 (2006).
Karlsson, E. et al. The mTOR effectors 4EBP1 and S6K2 are frequently coexpressed, and associated with a poor prognosis and endocrine resistance in breast cancer: a retrospective study including patients from the randomised Stockholm tamoxifen trials. Breast Cancer Res. 15, R96 (2013).
Cha, Y. L. et al. EIF4EBP1 overexpression is associated with poor survival and disease progression in patients with hepatocellular carcinoma. PLoS ONE 10, e0117493 (2015).
Miao, Y. et al. Increased phosphorylation of 4E-binding protein 1 predicts poor prognosis for patients with colorectal cancer. Mol. Med. Rep. 15, 3099–3104 (2017).
Kang, M. J. et al. 4E-BP is a target of the GCN2–ATF4 pathway during Drosophila development and aging. J. Cell Biol. 216, 115–129 (2017).
Yamaguchi, S. et al. ATF4-mediated induction of 4E-BP1 contributes to pancreatic β cell survival under endoplasmic reticulum stress. Cell Metab. 7, 269–276 (2008).
Pourdehnad, M. et al. Myc and mTOR converge on a common node in protein synthesis control that confers synthetic lethality in Myc-driven cancers. Proc. Natl Acad. Sci. USA 110, 11988–11993 (2013).
Ben-Sahra, I., Hoxhaj, G., Ricoult, S. J. H., Asara, J. M. & Manning, B. D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733 (2016).
Cunningham, J. T., Moreno, M. V., Lodi, A., Ronen, S. M. & Ruggero, D. Protein and nucleotide biosynthesis are coupled by a single rate-limiting enzyme, PRPS2, to drive cancer. Cell 157, 1088–1103 (2014).
Kirchner, S. et al. Alteration of protein function by a silent polymorphism linked to tRNA abundance. PLoS Biol. 15, e2000779 (2017).
Soufi, A., Donahue, G. & Zaret, K. S. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell 151, 994–1004 (2012).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Schmitz, R. et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N. Engl. J. Med. 378, 1396–1407 (2018).
Goldman, M. et al. The UCSC Cancer Genomics Browser: update 2015. Nucleic Acids Res. 43, D812–D817 (2015).
Acknowledgements
We thank D.M. Feldser for critically reading the manuscript. We thank the Koumenis and Maity laboratory members for helpful discussions. This work was supported by National Institutes of Health grants P01CA165997 (D.R., S.Y.F., J.A.D. and C.K.) and 5R01CA198015-04 to R.K.A. F.T. was supported by NIH F31CA183569.
Author information
Authors and Affiliations
Contributions
F.T. and C.K. conceived the experiments, analysed data and wrote the manuscript. F.T., I.I.V., N.M.L., R.O., C.S., F.C. and A.M.M. performed experiments. C.P. and Z.I. performed tRNA microarray and analysis. C.S.C., J.A.D., S.Y.F. and D.R. provided valuable reagents, important experimental suggestions and helped in manuscript editing. R.K.A. and J.Y. provided important experimental suggestions. A.K. provided bioinformatics support for ChIP–seq data analysis. W.F. and P.W. analysed patient datasets.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 GCN2 is required for optimal activation of ATF4.
a. Representative immunoblot following MYC activation in DLD-1, MycER cells (top) or MEFs, MycER (bottom). b. qPCR showing mRNA expression in GCN2 +/+ and GCN2 -/- : MycER MEFs (left) and DLD-1 MycER cells (right) normalized to 18s RNA. Three independent experiments, error bars represent mean ± SD, two tailed student t -test. c. Heat map of comparative microarray showing tRNA abundance following MYC activation. Data are relative to the control values. tRNA probes are depicted with their cognate codon and the corresponding amino acid; Meti, initiator tRNAMet, four biological replicates, one way-ANOVA, * p=0.018. d. Principal component analysis (PCA) of the changes in the tRNA abundance following MYC activation. All biological replicates (n=4) from tRNA microarrays (2h, 4h and 8h in panel c) were subjected in the analysis. tRNAs charged with different amino acids are color coded as follows: light gray, hydrophobic; dark gray, hydrophilic; blue, positively charged and red, negatively charged amino acids. e. Heat map of comparative microarrays showing tRNA charging following MYC activation in vehicle or 25μM RNA POLIII inhibitor (ML60218) treated cells. f. tRNA microarray of DLD-1: MycER cells pretreated for two hours with vehicle or ML60218 followed by 4hr of MYC induction showing tRNA abundance. Samples were normalized to vehicle treated cells. n=1. Immunoblot are from three independent experiments showing similar results. Unprocessed scans of blots are shown in Supplementary Fig 7.
Supplementary Figure 2 ATF4/MYC ChIP-seq.
a. Previously reported ATF4 targets occupied by ATF4 at 8hr of MYC induction within 5kb from TSS. b. Functions and pathways significantly enriched among genes bound by ATF4 within 5kb from TSS and upregulated at 8 hours of MYC activation, E=enrichment, FDR=false discovery rate, UP=Uniprot, MF=molecular function, BP=biological process. Two independent biological replicates were used for ChIP-seq showing similar result. P-values were calculated by Ingenuity Pathway Analysis using right-tailed Fisher Exact Test with FDR values indicating correction for multiple testing. c. ChIP-qPCR validation of ATF4 target genes (left) and genes bound by both ATF4 and MYC (right). Technical replicates, n=3.
Supplementary Figure 3 Antioxidants, fatty acids or alpha ketoglutarate do not rescue ATF4 deficient cells.
a. Representative immunoblot of ATF4 deficient MEFs treated with indicated antioxidants, Trolox and N-acetyl-cysteine (NAC) or Dimethyl 2-oxoglutarate (αKG) followed by MYC activation. b. Representative immunoblot of ATF4 deficient MEFs treated with indicated fatty acids followed by MYC activation. c. Representative immunoblot of MEFs treated with (αKG) at earlier time points of MYC activation. Immunoblots are representative of two independent experiments showing similar results. Unprocessed scans of blots are shown in Supplementary Fig 7.
Supplementary Figure 4 ATF4 and MYC cooperatively regulate EIF4EBP1.
a. qPCR showing expression of mRNAs in wild type and ATF4 -/-, MycER MEFs normalized to 18s RNA. b. qPCR showing expression of mRNAs in control and ATF4 knockdown DLD-1, MycER cells normalized to 18s RNA. a,b Three independent experiments, error bars represent mean ± SD, two tailed student t-test. c. Representative immunoblot of ATF4 -/-, MycER MEFs pretreated with indicated drugs for 2 hours prior to MYC activation. Rapamycin (Rapa) 200nM. Representative immunoblot from three independent experiments showing similar results. d. Clonogenic survival of ATF4 -/-, MycER MEFs after activation of MYC in the absence or presence of Rapamycin (200nM). Graph from there independent experiments, error bars represent mean ± SD, two tailed student t-test. e.35S incorporation in ATF4 -/-, MycER MEFs following MYC activation. Representative autoradiograph is shown. Graph data from three independent experiments, normalized to β-actin. Error bars represent mean ± SD, two tailed student t-test. f. ATF4-/-, MycER MEFs were pretreated with 5mM 4-Phenylbutyric acid (4PBA) for 2hrs followed by MYC activation. Representative western blot from two independent experiments is shown. g. Annexin V staining of cells transfected with the shown siRNAs, n=2, error bars represent mean ± SEM. Unprocessed scans of blots are shown in Supplementary Fig 7.
Supplementary Figure 5 Loss of GCN2 does not affect MYC driven lymphomagenesis but loss of GCN2 combined with inhibition of PERK promotes survival of MYC driven lymphoma bearing mice.
a. Representative images of genotyping PCR products of mice. PCR was performed more than three times independently. b. Kaplan-Meier analysis for overall survival of Eµ-Myc/+; Gcn2+/+ (n=28), Eµ-Myc/+; Gcn2+/- (n=27) and Eµ-Myc/+; Gcn2-/- mice(n=30). Kaplan-Meier curves were analyzed by two-tailed log-rank test. c. Representative western blot assessing ISR signaling in B cells isolated from tumor bearing mice or WT litter mates. Immunoblot is a representative of 3 independent experiments showing similar results. d. Schematic showing the treatment regimen performed in allograft model of lymphomagenesis. 2 million lymphoma cells were injected via tail vein into mice and LY-4 treatment was started three days after tumor injection. e. Body weight of mice injected with lymphoma cells during LY-4 treatment, (n=8 per each group). f. Pancreas weight of the mice in panel e. n=7 mice per group. Error bars represent mean ± SD, two tailed student t-test. g. Kaplan-Meier analysis for overall survival of mice treated with either vehicle or LY-4 (n=8 per each group). Kaplan-Meier curves were analyzed by two-tailed log-rank test. h. Representative immunoblot of ISR signaling examined in tumors isolated from the indicated groups in panel d at the end of the experiment. i. Quantification of immunoblots for p-eIF2α, including panel c. n = 3 for GCN2 +/+: Veh, n = 6 for GCN2 +/+: LY-4, n = 7 for GCN2 -/-: Veh and GCN2 -/-: LY-4. Error bars represent mean ± SD, two tailed student t-test. Unprocessed scans of blots are shown in Supplementary Fig 8. Unprocessed scans of blots are shown in Supplementary Fig 8.
Supplementary Figure 6 Acute deletion of ATF4 significantly delays MYC driven lymphomagenesis.
a. Schematic showing insertion of LoxP sites in mouse Atf4 locus. Exon 2 and 3, which code for ATF4 protein are deleted after Cre recombinase mediated excision. b. Genotyping PCR products of mice used in in vivo experiments. 1=Eμ-Myc/+, Rosa26CreERT2/+, ATF4+/+ 2= Eμ-Myc/+, Rosa26CreERT2/+, ATF4fl/fl, 3= WT. c. Kaplan-Meier analysis for lymphoma-free survival of mice bearing Eµ-Myc; ATF4+/+ lymphoma treated with either vehicle (veh) or tamoxifen (tam) for 5 days, n=10. Kaplan-Meier curves were analyzed by two-tailed log-rank test. d. mRNA expression of ATF4 from Fig 4d, showing a significant reduction of ATF4 mRNA in B cells treated with tamoxifen, n=3, mice per group. Error bars represent mean ± SD, two tailed student t-test. e. mRNA expression of ATF4 at the endpoint mice treated with vehicle or tamoxifen whose survival is shown in Fig 4b, c. n=3, mice per group. Error bars represent mean ± SD, two tailed student t-test.
Supplementary Figure 7
Unprocessed scans of western blots.
Supplementary information
Supplementary Information
Supplementary Figuress 1–7 and Supplementary Table titles and legends.
Supplementary Table 1
List of primers used.
Supplementary Table 2
List of antibodies used.
Supplementary Table 3
Statistics source data.
Rights and permissions
About this article
Cite this article
Tameire, F., Verginadis, I.I., Leli, N.M. et al. ATF4 couples MYC-dependent translational activity to bioenergetic demands during tumour progression. Nat Cell Biol 21, 889–899 (2019). https://doi.org/10.1038/s41556-019-0347-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-019-0347-9
This article is cited by
-
Protein lipidation in cancer: mechanisms, dysregulation and emerging drug targets
Nature Reviews Cancer (2024)
-
ATF4 in cellular stress, ferroptosis, and cancer
Archives of Toxicology (2024)
-
3D genomics and its applications in precision medicine
Cellular & Molecular Biology Letters (2023)
-
Characterization of endoplasmic reticulum stress unveils ZNF703 as a promising target for colorectal cancer immunotherapy
Journal of Translational Medicine (2023)
-
mTOR inhibition suppresses Myc-driven polyposis by inducing immunogenic cell death
Oncogene (2023)