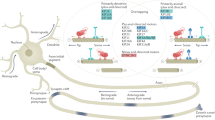
Abstract
Controlling cellular processes with light can help elucidate their underlying mechanisms. Here we present zapalog, a small-molecule dimerizer that undergoes photolysis when exposed to blue light. Zapalog dimerizes any two proteins tagged with the FKBP and DHFR domains until exposure to light causes its photolysis. Dimerization can be repeatedly restored with uncleaved zapalog. We implement this method to investigate mitochondrial motility and positioning in cultured neurons. Using zapalog, we tether mitochondria to constitutively active kinesin motors, forcing them down the axon towards microtubule (+) ends until their instantaneous release via blue light, which results in full restoration of their endogenous motility. We find that one-third of stationary mitochondria cannot be pulled away from their position and that these firmly anchored mitochondria preferentially localize to VGLUT1-positive presynapses. Furthermore, inhibition of actin polymerization with latrunculin A reduces this firmly anchored pool. On release from exogenous motors, mitochondria are preferentially recaptured at presynapses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Code availability
The MATLAB code used to analyse the data shown in Fig. 1c(iii) is available for download at https://github.com/ewest11/FTA-Fluorophore-Translocation-Analysis.
The ImageJ macro ‘Kymolyzer’ is available upon request.
References
Misgeld, T. & Schwarz, T. L. Mitostasis in neurons: maintaining mitochondria in an extended cellular architecture. Neuron 96, 651–666 (2017).
Schwarz, T. L. Mitochondrial trafficking in neurons. Cold Spring Harbor Perspect. Biol. 5, 1–15 (2013).
Sheng, Z.-H. & Cai, Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 13, 77–93 (2012).
Saxton, W. M. & Hollenbeck, P. J. The axonal transport of mitochondria. J. Cell. Sci. 125, 2095–2104 (2012).
Misgeld, T., Kerschensteiner, M., Bareyre, F. M., Burgess, R. W. & Lichtman, J. W. Imaging axonal transport of mitochondria in vivo. Nat. Methods 4, 559–561 (2007).
Voss, S., Klewer, L. & Wu, Y.-W. Chemically induced dimerization: reversible and spatiotemporal control of protein function in cells. Curr. Opin. Chem. Biol. 28, 194–201 (2015).
Wilson, M. H. & Holzbaur, E. L. F. Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells. Development 142, 218–228 (2015).
Jenkins, B., Decker, H., Bentley, M., Luisi, J. & Banker, G. A novel split kinesin assay identifies motor proteins that interact with distinct vesicle populations. J. Cell Biol. 198, 749–761 (2012).
del Castillo, U., Winding, M., Lu, W. & Gelfand, V. I. Interplay between kinesin-1 and cortical dynein during axonal outgrowth and microtubule organization in Drosophila neurons. Elife 4, e10140 (2015).
Komatsu, T. et al. Organelle-specific, rapid induction of molecular activities and membrane tethering. Nat. Methods 7, 206–208 (2010).
Tischer, D. & Weiner, O. D. Illuminating cell signalling with optogenetic tools. Nat. Rev. Mol. Cell Biol. 15, 551–558 (2014).
van Bergeijk, P., Adrian, M., Hoogenraad, C. C. & Kapitein, L. C. Optogenetic control of organelle transport and positioning. Nature 518, 111–114 (2015).
Czlapinski, J. L. et al. Conditional glycosylation in eukaryotic cells using a biocompatible chemical inducer of dimerization. J. Am. Chem. Soc. 130, 13186–13187 (2008).
Lester, H. A. & Nerbonne, J. M. Physiological and pharmacological manipulations with light flashes. Annu. Rev. Biophys. Bioeng. 11, 151175 (1982).
Banghart, M. R., He, X. J. & Sabatini, B. L. A caged enkephalin optimized for simultaneously probing mu and delta opioid receptors. ACS Chem. Neurosci. 9, 684–690 (2018).
Trigo, F. F., Corrie, J. E. & Ogden, D. Laser photolysis of caged compounds at 405 nm: photochemical advantages, localisation, phototoxicity and methods for calibration. J. Neurosc. Methods 180, 9–21 (2009).
Lemke, E. A., Summerer, D., Geierstanger, B. H., Brittain, S. M. & Schultz, P. G. Control of protein phosphorylation with a genetically encoded photocaged amino acid. Nat. Chem. Biol. 3, 769–772 (2007).
Gutnick, A., Banghart, M. R., West, E. R. & Schwarz, T. L. Synthesis of zapalog (TMP-DNAB-SLF). Protoc. Exch. https://doi.org/10.21203/rs.2.1716/v1 (2019).
Inoue, T., Heo, W., Grimley, J. S., Wandless, T. J. & Meyer, T. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat. Methods 2, 415–418 (2005).
Narendra, D., Tanaka, A., Suen, D.-F. & Youle, R. J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 (2008).
Tanaka, A. et al. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 191, 1367–1380 (2010).
Ashrafi, G., Schlehe, J. S., LaVoie, M. J. & Schwarz, T. L. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J. Cell Biol. 206, 655–670 (2014).
Lewis, T. L. & Polleux, F. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell 153, 1510–1525 (2013).
Chang, D. T. W., Honick, A. S. & Reynolds, I. J. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J. Neurosci. 26, 7035–7045 (2006).
Chada, S. R. & Hollenbeck, P. J. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr. Biol. 14, 1272–1276 (2004).
Ankenbruck, N., Courtney, T., Naro, Y. & Deiters, A. Optochemical control of biological processes in cells and animals. Angew. Chem. Int. Ed. 57, 2768–2798 (2018).
Khamo, J. S., Krishnamurthy, V. V., Sharum, S. R., Mondal, P. & Zhang, K. Applications of optobiology in intact cells and multicellular organisms. J. Mol. Biol. 429, 2999–3017 (2017).
Pathak, G. P., Strickland, D., Vrana, J. D. & Tucker, C. L. Benchmarking of optical dimerizer systems. ACS Synth. Biol. 3, 832–838 (2014).
van Bergeijk, P., Hoogenraad, C. C. & Kapitein, L. C. Right time, right place: probing the functions of organelle positioning. Trends Cell Biol. 26, 121–134 (2015).
Liu, P. et al. A bioorthogonal small-molecule-switch system for controlling protein function in live cells. Angew. Chem. Int. Ed. 53, 10049–10055 (2014).
Strickland, D. et al. TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat. Methods 9, 379–384 (2012).
Levskaya, A., Weiner, O. D., Lim, W. A. & Voigt, C. A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461, 997–1001 (2009).
Kennedy, M. J. et al. Rapid blue-light-mediated induction of protein interactions in living cells. Nat. Methods 7, 973–975 (2010).
Zimmermann, M. et al. Cell-permeant and photocleavable chemical inducer of dimerization. Angew. Chem. Int. Ed. 53, 4717–4720 (2014).
Lee, J.-R. et al. Characterization of the movement of the kinesin motor KIF1A in living cultured neurons. J. Biol. Chem. 278, 2624–2629 (2002).
Derr, N. D. et al. Tug-of-war in motor protein ensembles revealed with a programmable DNA origami scaffold. Science 338, 662–665 (2012).
Wang, X. & Schwarz, T. L. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell 136, 163–174 (2009).
Wang, X. et al. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147, 893–906 (2011).
Kang, J.-S. et al. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell 132, 137–148 (2008).
Pathak, D., Sepp, K. J. & Hollenbeck, P. J. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J. Neurosci. 30, 8984–8992 (2010).
Shepherd, G. M. & Harris, K. M. Three-dimensional structure and composition of CA3—>CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J. Neurosci. 18, 8300–8310 (1998).
Harris, K. M. & Weinberg, R. J. Ultrastructure of synapses in the mammalian brain. Cold Spring Harbor Perspect. Biol. 4, a005587 (2012).
Yi, M., Weaver, D. & Hajnóczky, G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J. Cell Biol. 167, 661–672 (2004).
Saotome, M. et al. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc. Natl Acad. Sci. USA 105, 20728–20733 (2008).
Al Awabdh, S. et al. Neuronal activity mediated regulation of glutamate transporter GLT‐1 surface diffusion in rat astrocytes in dissociated and slice cultures. Glia 64, 1252–1264 (2016).
Vale, R. D. The molecular motor toolbox for intracellular transport. Cell 112, 467–480 (2003).
Miyamoto, T. et al. Rapid and orthogonal logic gating with a gibberellin-induced dimerization system. Nat. Chem. Biol. 8, 465–470 (2012).
Kapitein, L. C. et al. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr. Biol. 20, 290–299 (2010).
Herzog, E. et al. In vivo imaging of intersynaptic vesicle exchange using VGLUT1 Venus knock-in mice. J. Neurosci. 31, 15544–15559 (2011).
Gornstein, E. L. & Schwarz, T. L. Neurotoxic mechanisms of paclitaxel are local to the distal axon and independent of transport defects. Exp. Neurol. 288, 153–166 (2017).
Pekkurnaz, G., Trinidad, J. C., Wang, X., Kong, D. & Schwarz, T. L. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell 158, 54–68 (2014).
Acknowledgements
We are grateful for plasmids shared with us by T. Inoue (Johns Hopkins University; Tom20–mCherry–FKBP), C. Hoogenraad (Utrecht University; PEX–mRFP–FKBP) and K. Verhey (U. Michigan; full-length Kif1a). We thank L. Mkhitaryan for technical support and the members of the T. L. Schwarz laboratory for fruitful discussions. We are grateful to S. Vasquez and M. Sahin’s laboratory for assistance with primary hippocampal cultures; D. Tom and L. Ding from Harvard NeuroDiscovery Center’s Enhanced Neuroimaging Core for assistance with live-cell imaging (NINDS P30 Core Center grant no. NS072030); and B. Sabatini (HMS) and G. Pekkurnaz (UCSD) for access to equipment. This research was generously supported by the National Institutes of Health grants R01 GM069808 (NIH/NIGMS) and R21 NS87582 (NIH/NINDS) to T.L.S. as well as a BCH Pilot Study Grant. A.G. was supported by F32 GM110984 (NIH/NIGMS) and T32 NS007484 (NIH/NINDS), and M.R.B. was supported by K99/R00 DA034648 (NIH/NIDA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A US patent for zapalog has been filed by Boston Children’s Hospital and approved (US10053445B2); T.L.S., M.R.B. and A.G. are listed as inventors.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Zapalog is photolysed by 405 nm, but not 458 nm light.
a, Liquid chromatography–mass spectrometry (LC-MS) of zapalog before and after photolysis (above- HPLC chromatogram, below- MS spectrogram of peaks found in HPLC). 1mM of zapalog in DMSO was analyzed by LC-MS and found to be highly pure, exhibiting a single peak at 11.96 min (compound A). Following exposure to 405 nm laser light, compound A is photocleaved into compounds B and C. The expected nitrosyl ketone C was observed along with another photoreaction product of putative structure D. b, Chromatograms of zapalog before and after exposure to 458 nm light. 1mM of zapalog in DMSO was exposed to 458 nm laser light on a confocal microscope (~100 µW for 1 min), then analyzed by HPLC. Zapalog was found to be insensitive to 458 nm light.
Supplementary Figure 2 Synthesis of zapalog (TMP-DANB-SLF).
A description of each intermediate reaction is presented in Methods.
Supplementary Figure 3 Tethering constitutively active motors to axonal mitochondria with zapalog forces most, but not all, mitochondria towards axon tips.
a, Zapalog dimerizes constitutively active kinesin motor Kif1a(1–489)-DHFR-myc to mitochondria tagged with Tom20-mCherry-FKBP. Representative images of immunocytochemistry with an antibody directed against myc-tagged constitutively active kinesin motor Kif1a(1–489)-DHFR-Myc (green) in DIV10 E18 cultured hippocampal neurons treated for 5 min with 1.5 µM of either DMSO or zapalog, in the dark. Zapalog, but not DMSO, causes the exogenous motors to translocate to the outer surface of all mitochondria. (n = 7 axons). b, A representative kymograph of a section of distal axon of an E18 rat hippocampal neuron that had been transfected with mitochondrial Tom20-mCherry-FKBP and the constitutively active kinesin motor Kif1a(1–489)-DHFR-myc. 1. Endogenous motility consists of mitochondria that are either stationary or motile in the retrograde and anterograde directions. 2. Addition of zapalog induces attachment of motors to mitochondria and subsequently most, but not all mitochondria are dragged in the anterograde direction. 3. Exposure to 405 nm light causes the exogenous motors to detach completely from mitochondria, resulting in immediate stoppage of some dragged mitochondria and resumption of bidirectional motility of others. Representative traces of individual mitochondria that were found to be immovable by zapalog-tethered motors in this assay(red), stationary but movable (yellow) and motile (green). c, Tethering mitochondria to Kinesin motors with zapalog causes them to pile-up at growth cones. Kymograph and sample time-lapsed images of the growth cone of an E18 rat hippocampal neuron that had been transfected with mitochondrial Tom20-mCherry-FKBP and the constitutively active kinesin motor Kif1a(1–489)-DHFR-myc. Addition of zapalog induced attachment of the motors to axonal mitochondria, forced mitochondrial motility towards the (+) end of microtubules, and resulted in the accumulation of mitochondria at axonal growth cones. Photolysis of zapalog released the motors, preventing additional mitochondrial translocation, but the mitochondria piled up at the growth cone do not recover motility and move retrograde. When similar aggregations formed along axons, the aggregates did not disperse upon photolysis of zapalog and the affected axons often degenerated. d, Long term flux analysis of zapalog-mobilized mitochondria reveals depletion of mitochondria from a region of axon. (top) A micrograph depicting a field with two intersecting axons of E18 rat hippocampal neurons, traced in red and blue, that had been transfected with mitochondrial Tom20-mCherry-FKBP and the constitutively active kinesin motor Kif1a(1–489)-DHFR-Myc, in the presence of 1.5 µM zapalog. The arrows indicate the +-end directed flow of mitochondria driven by the Kif1a motor. (bottom) Simultaneous flux analysis of these axons demonstrates that forced mobilization of axonal mitochondria peaks at different times at 40–50 mitochondria per second and then subsequently decreases as movable mitochondria are eventually depleted from the upstream, proximal regions of axon, leaving only the anchored pool. Distal regions, and especially growth cones are correspondingly enriched, as in c. Source data are available online .
Supplementary Figure 4 Latrunculin A eliminates detectable filamentous actin but does not detectably alter axonal tubulin and VGLUT1.
Representative images of axons from DIV10 E18 cultured hippocampal neurons treated for 6 hrs with 2.5 μM of either DMSO or Latrunculin A. Axons were labeled with antibodies directed against VGLUT1 (red), neuron-specific class III β-tubulin (TUJ1, green), and F-actin was visualized by staining with Alexa Fluor 647 phalloidin (greyscale). (scale bar = 10 μm, n = 15 neurons each).
Supplementary Figure 5 An automated pipeline for quantifying fluorophore translocation.
a, Representative micrograph from a time-lapsed Fluorophore Translocation Assay of a COS7 cell transfected with cytoplasmic YFP-DHFR-Myc (cyan) and mitochondrial Tom20-mCherry-FKBP (red). b, Each cell’s outline is thresholded using the cytoplasmic YFP-DHFR-Myc channel before zapalog addition. c, Each cell’s mitochondria are separately thresholded for every timepoint using the mitochondrial Tom20-mCherry-FKBP channel. d, At each timepoint, the mitochondrial fill (c) is subtracted from the total cell fill (b) to calculate the area of the cell that is only cytoplasmic and not mitochondrial. e, The ratio of mitochondrial/cytoplasmic YFP is plotted over time, from immediately following zapalog addition until after zapalog photolysis.
Supplementary Figure 6 Quantification of stationary mitochondria at presynaptic sites and generation of random positions along the axon for use as controls.
a, A representative example of how colocalization of stationary mitochondria and stationary VGLUT1 signals was scored. Stationary mitochondria in kymographs were marked with 5 pixel-wide vertical lines (yellow). The marked lines were then superimposed onto the VGLUT1 channel and scored for coincidence with the stationary VGLUT1-Venus signal. b, A representative example of random position generation along the axon. For each dataset we generated a set of random positions along the axon equal in number to the number of capture events recorded for that dataset. These were plotted as 5 pixel-wide lines (same width as lines drawn to score data in a), overlapped with the dataset and scored similar to the experimental data. To this end, using Apple Numbers, we generated a list of random numbers for each dataset, with each number valued between 1 and the pixel width of the corresponding dataset (in this case 1000 pixels); the length of each list was also set to equal the number of stationary mitochondria found in the corresponding dataset (table 1). We then matched the randomly generated numbers with cells on a 1–1000 table, assigned the corresponding row a value of 100 and plotted as a histogram with 1 pixel-wide bars. We also assigned a value of 100 to the 2 rows before and 2 rows after each random number, widening the bars to 5 pixels in length (table 2). Finally, we superimposed the histogram onto the corresponding kymograph and proceeded to score as shown in a.
Supplementary information
Supplementary Information
Supplementary Figures 1–6, Supplementary Table and Video titles/legends.
Supplementary Table 1
Statistics source data.
Supplementary Video 1
Zapalog induced translocation of YFP from cytoplasm to mitochondria.
Supplementary Video 2
Selective removal of YFP from mitochondria with zapalog and targeted 405 nm light.
Supplementary Video 3
Repeated rounds of translocation and removal of YFP on and off of a mitochondrion with zapalog and targeted light.
Supplementary Video 4
Repeated rounds of translocation and removal of YFP on and off of peroxisomes with zapalog and targeted light.
Supplementary Video 5
Mitochondria are driven towards axon tips by zapalog-dependent tethering to Kif1a motors, then instantaneously released by light.
Supplementary Video 6
Mitochondria accumulate at the distal tip of an axon after being tethered to Kif1a motors by zapalog.
Supplementary Video 7
Zapalog-induced mobilization of axonal mitochondria.
Rights and permissions
About this article
Cite this article
Gutnick, A., Banghart, M.R., West, E.R. et al. The light-sensitive dimerizer zapalog reveals distinct modes of immobilization for axonal mitochondria. Nat Cell Biol 21, 768–777 (2019). https://doi.org/10.1038/s41556-019-0317-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-019-0317-2
This article is cited by
-
Small molecule-nanobody conjugate induced proximity controls intracellular processes and modulates endogenous unligandable targets
Nature Communications (2023)
-
Fluorogenic chemically induced dimerization
Nature Methods (2023)
-
A fluorogenic chemically induced dimerization technology for controlling, imaging and sensing protein proximity
Nature Methods (2023)
-
Energy matters: presynaptic metabolism and the maintenance of synaptic transmission
Nature Reviews Neuroscience (2022)
-
Mitochondrial heterogeneity and homeostasis through the lens of a neuron
Nature Metabolism (2022)