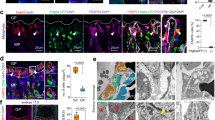
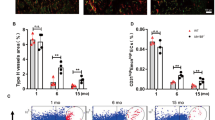
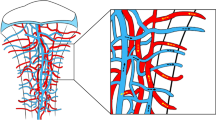
Abstract
Growth plate cartilage contributes to the generation of a large variety of shapes and sizes of skeletal elements in the mammalian system. The removal of cartilage and how this process regulates bone shape are not well understood. Here we identify a non-bone-resorbing osteoclast subtype termed vessel-associated osteoclast (VAO). Endothelial cells at the bone/cartilage interface support VAOs through a RANKL–RANK signalling mechanism. In contrast to classical bone-associated osteoclasts, VAOs are dispensable for cartilage resorption and regulate anastomoses of type H vessels. Remarkably, proteinases including matrix metalloproteinase-9 (Mmp9) released from endothelial cells, not osteoclasts, are essential for resorbing cartilage to lead directional bone growth. Importantly, disrupting the orientation of angiogenic blood vessels by misdirecting them results in contorted bone shape. This study identifies proteolytic functions of endothelial cells in cartilage and provides a framework to explore tissue-lytic features of blood vessels in fracture healing, arthritis and cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-seq data that support the findings of this study have been deposited in the Array Express database under accession code E-MTAB-6857. Numerical source data giving rise to graphical representations and statistical descriptions have been provided as Supplementary Table 2. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Kronenberg, H. M. Developmental regulation of the growth plate. Nature 423, 332–336 (2003).
Mackie, E. J., Ahmed, Y. A., Tatarczuch, L., Chen, K. S. & Mirams, M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 40, 46–62 (2008).
Bolander, M. E. Regulation of fracture repair by growth factors. Proc. Soc. Exp. Biol. Med. 200, 165–170 (1992).
Einhorn, T. A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 355, S7–S21 (1998).
Chan, C. K. et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457, 490–494 (2009).
Reddi, A. H. & Anderson, W. A. Collagenous bone matrix-induced endochondral ossification hemopoiesis. J. Cell Biol. 69, 557–572 (1976).
Lewinson, D. & Silbermann, M. Chondroclasts and endothelial cells collaborate in the process of cartilage resorption. Anat. Rec. 233, 504–514 (1992).
Boyle, W. J., Simonet, W. S. & Lacey, D. L. Osteoclast differentiation and activation. Nature 423, 337–342 (2003).
Henriksen, K., Bollerslev, J., Everts, V. & Karsdal, M. A. Osteoclast activity and subtypes as a function of physiology and pathology–implications for future treatments of osteoporosis. Endocr. Rev. 32, 31–63 (2011).
Hunziker, E. B. & Schenk, R. K. Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone growth in rats. J. Physiol. 414, 55–71 (1989).
Gerber, H. P. et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 5, 623–628 (1999).
Kanczler, J. M. & Oreffo, R. O. Osteogenesis and angiogenesis: the potential for engineering bone. Eur. Cells Mater. 15, 100–114 (2008).
Schipani, E., Maes, C., Carmeliet, G. & Semenza, G. L. Regulation of osteogenesis–angiogenesis coupling by HIFs and VEGF. J. Bone Miner. Res. 24, 1347–1353 (2009).
Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328 (2014).
Ramasamy, S. K., Kusumbe, A. P., Wang, L. & Adams, R. H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507, 376–380 (2014).
Ramasamy, S. K. et al. Blood flow controls bone vascular function and osteogenesis. Nat. Commun. 7, 13601 (2016).
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Fantin, A. et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829–840 (2010).
Davies, L. C., Jenkins, S. J., Allen, J. E. & Taylor, P. R. Tissue-resident macrophages. Nat. Immunol. 14, 986–995 (2013).
Fong, T. A. et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 59, 99–106 (1999).
Itkin, T. et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328 (2016).
Kusumbe, A. P. et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380–384 (2016).
Wang, Y. et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 (2010).
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Yasuda, H. et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl Acad. Sci. USA 95, 3597–3602 (1998).
Xiong, J. et al. Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241 (2011).
Fleisch, H. Bisphosphonates: mechanisms of action. Endocr. Rev. 19, 80–100 (1998).
Hughes, D. E. et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res. 10, 1478–1487 (1995).
Kodama, H. et al. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J. Exp. Med. 173, 269–272 (1991).
Marks, S. C. Jr. Morphological evidence of reduced bone resorption in osteopetrotic (op) mice. Am. J. Anat. 163, 157–167 (1982).
Vu, T. H. et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93, 411–422 (1998).
Breur, G. J., VanEnkevort, B. A., Farnum, C. E. & Wilsman, N. J. Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. J. Orthop. Res. 9, 348–359 (1991).
Cooper, K. L. et al. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 495, 375–378 (2013).
Farnum, C. E., Tinsley, M. & Hermanson, J. W. Forelimb versus hindlimb skeletal development in the big brown bat, Eptesicus fuscus: functional divergence is reflected in chondrocytic performance in autopodial growth plates. Cells Tissues Organs 187, 35–47 (2008).
Wilsman, N. J., Bernardini, E. S., Leiferman, E., Noonan, K. & Farnum, C. E. Age and pattern of the onset of differential growth among growth plates in rats. J. Orthop. Res. 26, 1457–1465 (2008).
Wilsman, N. J., Farnum, C. E., Leiferman, E. M., Fry, M. & Barreto, C. Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J. Orthop. Res. 14, 927–936 (1996).
Marenzana, M. & Arnett, T. R. The key role of the blood supply to bone. Bone Res. 1, 203–215 (2013).
Lee, S. et al. Intermittent PTH 1-34 administration improves the marrow microenvironment and endothelium-dependent vasodilation in bone arteries of aged rats. J. Appl. Physiol. 124, 1426–1437 (2018).
Kroll, M. H. Parathyroid hormone temporal effects on bone formation and resorption. Bull. Math. Biol. 62, 163–188 (2000).
Ferguson, C., Alpern, E., Miclau, T. & Helms, J. A. Does adult fracture repair recapitulate embryonic skeletal formation? Mech. Dev. 87, 57–66 (1999).
Gerstenfeld, L. C. et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J. Bone Miner. Res. 18, 1584–1592 (2003).
Goldring, M. B. Osteoarthritis and cartilage: the role of cytokines. Curr. Rheumatol. Rep. 2, 459–465 (2000).
Goldring, S. R. Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology 42, ii11–ii16 (2003).
Carter, R. L. & Tanner, N. S. Local invasion by laryngeal carcinoma–the importance of focal (metaplastic) ossification within laryngeal cartilage. Clin. Otolaryngol. Allied Sci. 4, 283–290 (1979).
Castelijns, J. A. et al. Invasion of laryngeal cartilage by cancer: comparison of CT and MR imaging. Radiology 167, 199–206 (1988).
Lubberts, E. et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 50, 650–659 (2004).
Zwerina, J. et al. Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum. 50, 277–290 (2004).
Takeshita, S., Kaji, K. & Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 15, 1477–1488 (2000).
Xie, H. et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 20, 1270–1278 (2014).
Maes, C. et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech. Dev. 111, 61–73 (2002).
Koch, U. et al. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J. Exp. Med. 205, 2515–2523 (2008).
Hoeck, J. D. et al. Fbw7 controls neural stem cell differentiation and progenitor apoptosis via Notch and c-Jun. Nat. Neurosci. 13, 1365–1372 (2010).
Haase, V. H., Glickman, J. N., Socolovsky, M. & Jaenisch, R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc. Natl Acad. Sci. USA 98, 1583–1588 (2001).
Kusumbe, A. P., Ramasamy, S. K., Starsichova, A. & Adams, R. H. Sample preparation for high-resolution 3D confocal imaging of mouse skeletal tissue. Nat. Protoc. 10, 1904–1914 (2015).
Liao, Y., Smyth, G. K. & Shi, W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 41, e108 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Acknowledgements
We thank A. Duarte, F. Radtke and A. Behrens for floxed Dll4 and Fbxw7 mutant mice. We thank L. Game and I. Andrew from the LMS Genomics Facility. We thank G. Dharmalingam from LMS Bioinformatics for helping with R analysis. We also thank the LMS/NIHR Flow Facility and CBS at Imperial College London for technical support. We thank P. Xi for mice injections. S.K.R. is a Sir Henry Dale Fellow of the Wellcome Trust and the Royal Society (202300/Z/16/Z). Funding to S.K.R. was provided by the Wellcome Trust, the Royal Society, the Medical Research Council and the American Bone and Mineral Research Society. A.P.K. is supported by the Medical Research Council (CDA- MR/P02209X/1), the Kennedy Trust for Rheumatology Research (AZR00700) and Leuka.
Author information
Authors and Affiliations
Contributions
S.G.R. and K.M.A. designed and performed most of the experiments and interpreted results. J.R. performed immunostaining and generated confocal images. A.S. generated RNA-seq data. A.P.K. provided tools, designed experiments and interpreted results. All of the authors prepared the manuscript. S.K.R. conceived the study, designed and performed experiments, interpreted results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Osteoclast subtypes in bone.
a, Tile scan image shows localisation of osteoclasts in a 4 weeks old mouse tibia. TRAP (osteoclasts, green) immunostaining indicates the distribution of osteoclasts in this postnatal bone. DAPI is nuclear counterstain. Scale bar, 500μm. Representative image from 3 repeated experiments. b, Quantification of total osteoclasts present in tibia of a 4-week old mouse. MP, metaphysis; DP, diaphysis. Data represent mean ± sem from n = 7 biologically independent samples. P values, two-tailed unpaired t-tests. c, Tile scan shows distribution of Vpp3 + osteoclasts (green) in a 4-weeks old mouse tibia from 8 repeated experiments. Blood vessels were immunostained with Endomucin (Emcn, red). mp, metaphysis; dp, diaphysis. Scale bar, 300 μm. d, Tibia from double transgenic mice generated using Csf1r Mer-iCre-Mer and R26-tdTomato were analysed for Tomato expression after tamoxifen induction. Experimental schedule of tamoxifen injection and analysis are as shown. Tomato+ large cells present in the metaphysis and diaphysis coincides with the osteoclasts markers expression in mouse tibia as observed from 10 repeated experiments. Scale bar, 100 μm (metaphysis), 50 μm (diaphysis). e, Osteoclast markers expression in 4 weeks old mouse tibia. RANK (green) and Emcn (red) immunostaining in (i), Cathepsin K (green) and DAPI (blue) in (ii), and Chloride voltage gated channel-7 (green) and DAPI (blue) in (iii) show similar distribution pattern of osteoclasts in the metaphysis of tibia. Endomucin (Emcn) marks blood vessels. DAPI is nuclear counterstain. Representative images from 4 repeated experiments. Scale bar, 50 μm. f-g, Pro-angiogenic macrophage markers expression in leading vascular front. f, Neuropilin1 (Nrp1, green) is not found to be expressed in osteoclasts (Itgb3, magenta). g, Tie2 (green) expression is detected only in blood vessels in the metaphysis. Emcn (red) labels blood vessels. Representative images from 4 repeated experiments. Scale bars, 50 μm, inset 8 μm. h, Graph shows percentage of osteoclasts occupying proximal and distal metaphysis of 3 weeks old mouse tibia. Data represent mean ± sem from n = 6 biologically independent samples. P values, two-tailed unpaired t-tests. i, A single low-dose tamoxifen injection to genetically label and understand relation between VAO and BAO subsets using double transgenic Csf1r Mer-iCre-Mer and R26-tdTomato mice. Tomato expression (red) was analysed after 6, 12, 24, 48 and 120 h post induction. Itgb3 immunostaining (green) was performed to label osteoclasts. Scale bar, 50μm. Representative images from 3 repeated experiments. j, Liposome encapsulated DiI dye injection showed localisation of DiI in phagocytes and a few cells in the metaphysis by 6 h. Both VAO and BAO subsets were labelled by 24 h. Blood vessels (Emcn, red); Phagocytic cells (Liposome DiI, White). Scale bar, 50μm. Representative images from 4 repeated experiments. k, Representative flow cytometry plots demonstrate the gating strategy used to purify osteoclasts from erythrocytes-lysed total bone cells. First, RANK + cells were gated out from total bone marrow cells. Then these cells were further separated on the basis of their nuclear content i.e cells with 2 nuclei were separated as VAO population compared cells BAO subset having > 4 nuclei.
Supplementary Figure 2 Distribution of osteoclasts and their association with blood vessels.
a-c, Tile scan images show distribution of osteoclasts (Itgb3, magenta) in various bone tissues such as calvarium (a), Vertebrum (b) and sternum (c) of 4-week-old mice. Blood vessels were immunostained using endomucin (Emcn, red) and CD31(green). DAPI stains nuclei. Scale bars, 200 μm (a,b), 100 μm (c). Representative images from 3 repeated experiments. d, Osteoclasts (Itgb3, green) in the cortical region of tibia are large elongated structures similar to BAO. Despite their proximity to blood vessels (Emcn, red), they seem to stretch along the bone surface. Scale bar, 50 μm. Representative images from 8 repeated experiments. e, Graph shows percentage variation of BAO and VAO subsets during bone growth. Developing bone contains predominantly VAO subset which decreases with age that lead to majority of BAO subset in adults. Data represent mean ± sem from n = 6 animals pooled from 3 experiments. f, Quantification of total osteoclasts number in the tibial metaphysis of control and vegfr2 inhibitor (SU5416) treated mice. Data represent mean ± sem from n = 6 biologically independent samples. P values, two-tailed unpaired t-tests. g, Confocal images showing metaphysis of control and Vegfr2 inhibitor (SU5416) treated mouse tibia immunostained for blood vessels (Emcn, red) and osteoclasts (Itgb3, green) at 4 weeks of age. Scale bar, 50μm. Representative images from 6 experimental animals. h, Vegfr2 treatment resulted in dramatic reduction in percentage of VAO subset. Data represent mean ± sem from n = 6 biologically independent samples pooled from 4 experiments. P values, two way-ANOVA with Sidak’s multiple comparisons test. i-j, Tibia from double transgenic mice generated using Cdh5 (PAC) CreERT2 and R26 lox-STOP-lox tdTomato transgenic background were analysed for Tomato expression after tamoxifen induction. Tamoxifen induces tomato expression in cells which undergo Cre mediated recombination. Thick bone sections were analysed for tomato expression and immunostained for various cell markers to understand the specificity of the Cre driver. i, Confocal images show blood vessel specific recombination in metaphysis and diaphysis of bone. Endomucin immunostaining (emcn, green) coincides with the endogenous tomato expression (red). j, Confocal images show Tomato expression (red) along with other cell type specific markers; CD45 labels haematopoietic cells, Osterix (Osx) marks osteoprogenitors, Sox9 was used to label chondrocytes and Itgb3 shows distribution of osteoclasts. Tomato expression does not overlap with any of these cell types. This also indicates the specificity of this Cre driver for blood vessels in bone. Scale bar, 50 μm. Representative images from 10 repeated experiments.
Supplementary Figure 3 Type-H vessels support VAO subtype of osteoclasts.
a, Representative images show arrangement of blood vessels (Emcn, red) and osteoclasts (Itgb3, green) in the metaphysis of 5-week-old Dll4 iΔEC mutants and control littermates from n = 4 independent experiments. Dapi, Nuclei. Scale bar, 50 μm. Graphs show osteoclasts number in Dll4 iΔEC mutants and their littermate controls. Total number of osteoclasts is not affected in endothelial specific deletion of Dll4. Data represent mean ± sem from n = 12 animals pooled from 4 independent experiments. P values, two-tailed unpaired t-tests. However, quantification of VAO and BAO osteoclast subtypes in the metaphysis of Dll4 iΔEC mutants and their control littermates shows reduction in ECs coordinated with the decline in VAO and increase in BAO subtypes. Data represent mean ± sem from n = 12 animals pooled from 4 independent experiments. P values, two way-ANOVA with Sidak’s multiple comparisons test. b, Representative confocal images showing arrangement of blood vessels (Emcn, red) and osteoclasts (Itgb3, green) in the metaphysis of Fbxw7 iΔEC mutants and their littermate controls from 4 independent experiments. Scale bar, 50 μm. Quantification of total osteoclasts shows similar number in Fbxw7 iΔEC mutants and their littermate controls. Data represent mean ± sem from n = 12 animals pooled from 4 independent experiments. P values, two-tailed unpaired t-tests. However, Quantification of VAO and BAO osteoclast subtypes in the metaphysis of Fbxw7 iΔEC mutants and their control littermates show increase in VAO subset upon promotion of ECs in Fbxw7 iΔEC animals. Data represent mean ± sem from n = 12 animals pooled from 4 independent experiments. P values, two way-ANOVA with Sidak’s multiple comparisons test. Quantification of VAO subset indicates the increase in VAO is prominent in the distal metaphysis. Data represent mean ± sem from n = 7 samples pooled from 4 independent experiments. P values, two way-ANOVA with Sidak’s multiple comparisons test. c, Experimental scheme shows tamoxifen injection schedule used to induce gene deletion in aged Vhl mice. Representative confocal images show reactivation of type-H vessels (yellow) in Vhl iΔEC mutants compared to their aged control littermates (Vhl p/p Cre-) from n = 3 independent animals. Type-H vessels show high expression of endothelial markers CD31 (green) and Emcn (red). Reactivation of type-H vessels is associated with increase in osteoclasts (Itgb3, white) in the metaphysis. White arrows indicate osteoclasts in close association with type-H vessels while blue arrow indicates an osteoclast not associated with blood vessels. Scale bar, 50 μm. Quantification of blood vessel associated osteoclasts (VAOs) show increase in their number in Vhl iΔEC mutants compared to their aged control littermates. Data represent mean ± sem from n = 3 biologically independent experiments. P values, two-tailed unpaired t-tests.
Supplementary Figure 4 Growth plate analysis upon defective blood vessel growth.
a, Tile scan images show TUNEL + cells (green) in the growth plates of Dll4 iΔEC mutants and their littermate controls of 10-weeks old. DAPI is nuclear counterstain. Scale bars, 200 μm. Representative image from 6 biologically repeated experiments. b, Graph shows growth plate sizes of endothelial Dll4 mutants and their littermate controls. Data represent mean ± sem from n = 6 biologically independent experiments. P values, two-tailed unpaired t-tests. c, Quantification of TUNEL + cells in the growth plate region of Dll4 iΔEC and corresponding controls show increase in TUNEL + cells in the mutant animals, Data represent mean ± sem from n = 10 bones pooled from 4 experiments. P values, two-tailed unpaired t-tests. d, Quantification of cleaved caspase3 + cells in control and vegfr2 inhibitor (SU5416) treated mice. Data represent mean ± sem from n = 6 biologically independent experiments. P values, two-tailed unpaired t-tests. e, Representative images show Collagen-X and MMP13 expression in the growth plates of 4-week-old control and Vegfr2 inhibitor (SU5416) treated mice from 6 biologically independent animals. Scale bars, 50 μm. f, Tile scan image show Collagen-X (ColX, green) expression in the growth plates of Dll4 iΔEC mutants and littermate controls. Scale bars, 150 μm. Maximum intensity projection images show MMP13 (green) and Endomucin (red) in the growth plates of Dll4 iΔEC mutants and littermate controls. Scale bars, 50μm. DAPI stains nuclei. Both are representative images from 6 biologically independent animals. g, Graphs show Rankl transcript levels in various cell types in both Rankl iΔEC mutants and their littermate controls. Total RNA isolated from purified endothelial cells of n = 8 biologically independent animals, chondrocytes of n = 4 biologically independent animals, osteoblasts/osteocytes of n = 4 biologically independent animals and haematopoietic cells of n = 4 biologically independent animals were subjected to reverse transcription and quantitative PCR to analyse Rankl levels. Significant decrease in Rankl transcripts were observed in bone endothelial cells while other cell types did not show any change. This confirms the endothelial specific deletion of Rankl in these mutants. Data represent mean ± sem. P values, two-tailed unpaired t-tests.
Supplementary Figure 5 Interaction between blood vessels and osteoclasts.
a, Tile scan images of Rankl iΔEC mutant and littermate control mice tibia immunostained for Osteopontin (green) display increased bone matrix in the mutant animals. DAPI is nuclear counterstain. Scale bars, 500 μm. Representative images from 4 biologically independent animals. b, Confocal image of Rankl iΔEC mutant and littermate control mice tibial metaphysis show distribution of osteoprogenitors (Osx, green). Blood vessels were immunostained with Emcn (red). Scale bars, 50 μm. Representative images from 6 biologically independent animals. c, Graphs show quantification of fold change in number of type-H (CD31high/Emcnhigh) and type-L (CD31low/Emcnlow) endothelial cells in Rankl iΔEC mutants and their littermate controls on P28. Data represent mean ± sem from n = 5 biologically independent animals. P values, two-tailed unpaired t-tests. d, Confocal images of Rankl iΔEC mutants and their littermate control mice show status of type-H endothelial cells in 4 weeks old tibia. Type-H endothelial cells were identified using high expression of CD31 (green) and Emcn (red) markers. Scale bars, 50 μm. Representative images from 6 biologically independent animals. e, Liposome encapsulated DiI dye injection showed localisation of phagocytic cells in tibia of Rankl iΔEC mutants and their littermate controls after 24 h post-injection. Both VAO and BAO subsets were labelled in control mice. Reduced VAO number in Rankl iΔEC mutants led to decreased DiI labelling in proximal metaphysis. Blood vessels (Emcn, red); Phagocytic cells (Liposome DiI, White). Scale bars, 50 μm. Representative images from 3 biologically independent animals. Quantification of DiI labelled cells indicate decreased phagocytic cells in the proximal metaphysis of Rankl iΔEC mutants. Data represent mean ± sd from n = 7 biologically independent animals. P values, two-tailed unpaired t-tests. f, Confocal images show growth plates of Rankl iΔEC mutant and littermate control mice tibia immunostained for Collagen-X and MMP13 (green). DAPI stains nucleus. Hypertrophic zone in the growth plate is not affected in the mutant animals. Scale bars, 50 μm. Representative images from n = 3 biologically independent animals. Quantification of cleaved caspase3 + cells in Rankl iΔEC mutant and littermate control mice. Data represent mean ± sd from n = 6 biologically independent animals. P values, two-tailed unpaired t-tests.
Supplementary Figure 6 Growth plate analysis and blood vessel orientations in bone.
a, Tile scan images immunostained for Osteopontin (red) indicate increased bone formation in the alendronate treated animals compared to their control. DAPI is nuclear counterstain. Scale bars, 500 μm (control), 300 μm (alendronate). Representative images from 4 repeated experiments. b, Quantification of osteoclasts show reduced number in the alendronate treated animals in comparison to their controls. Data represent mean ± sem from n = 11 animals pooled from 4 independent experiments. P values, two-tailed unpaired t-tests. Further quantifying both VAO and BAO subsets indicate that alendronate treatment affects both the subtypes. Data represent mean ± sem from n = 11 animals pooled from 4 independent experiments. P values, two way-ANOVA with Sidak’s multiple comparisons test. c, Confocal images of Collagen-X (ColX, green) immunostained growth plates in control and alendronate treated animals show unaffected hypertrophic zones. DAPI is nuclear counterstain. Scale bars, 50 μm. Representative images from 3 repeated experiments. d, Quantification of osteoclasts number in tibia after short-term treatment of alendronate. Alendronate administration at 7th day shows reduction in VAO subtypes while BAO subtypes were not significantly affected by the treatment. Data represent mean ± sem from n = 6 biologically independent animals. P values, two way-ANOVA with Sidak’s multiple comparisons test. e, Graph showing Mmp9 transcript levels in purified endothelial cells of Mmp9 iΔEC mutants and their littermate controls indicates reduction of Mmp9 levels after endothelial specific deletion. Data represent mean ± sd from n = 4 biologically independent experiments. P values, two-tailed unpaired t-tests. f, Quantification of cleaved caspase3 + cells in control and Mmp9 iΔEC mutants indicate increase in activated caspase3 + cells upon endothelial specific deletion of Mmp9. Data represent mean ± sem from n = 6 biologically independent experiments. P values, two-tailed unpaired t-tests. g, Confocal images show growth plates of Mmp9 iΔEC mutant and littermate control mice tibia immunostained for MMP13 (green). DAPI stains nucleus. Scale bars, 50 μm. Representative images from 4 repeated experiments. h, Quantification of osteoclasts show a comparable number in osteoclast specific Mmp9 deletion mutants in comparison to their littermate controls. Data represent mean ± sd from n = 5 animals pooled from 2 experiments. P values, two-tailed unpaired t-tests. i, Quantifications to show direction of type-H and type-L vessels and chondrocytes in bone. Percentage of blood vessel structures and columnar chondrocytes were plotted based on their angular orientation along the distal-proximal axis of tibia. Angular orientation of blood vessel structures in secondary ossification centre (SOC) were measured along the radial axis of bone transverse sections. In long bones. type-H vessels are arranged along the direction of bone growth while columnar chondrocytes in growth plate expand in parallelly opposite direction. Type-L vessels are generally in perpendicular orientation. In SOCs, type-H vessels do not show any particular orientation and their broad orientations indicate their growth in all the directions. Data represent mean ± sd from n = 5 biologically independent samples. P values, two-tailed unpaired t-tests.
Supplementary Figure 7 Relation between blood vessel orientation and bone growth.
a, Tile scan confocal image illustrates type-H vessels arrangement in femur head. Type-H vessels identified by CD31 (green) and Emcn (red) high expression is localised near cartilage region. White arrows indicate the direction of blood vessel growth. Osteoclasts (Itgb3, magenta) show similar distribution pattern as observed in tibia. DAPI is nuclei counterstain. Scale bars, 100 μm. Representative image from 2 repeated experiments. b, Confocal images show cartilage islands formed in Dll4 iΔEC mutants treated with alendronate caused by growth of randomly directed blood vessels. Itgb3, osteoclasts (green); Emcn, blood capillaries (red); DAPI, Nuclei (blue). Scale bars, 50 μm. Representative images from 4 repeated experiments. c, Experimental scheme illustrating the tamoxifen administration and PTH treatment in Dll4 transgenic animals to investigate their function in blood vessels and directional bone growth. d, Schematic illustration highlighting the behaviour of type-H capillaries in each treatment conditions that first misdirect and then promote blood vessel growth. e, Confocal tile scan images show status and distribution of type-H blood vessels (yellow) identified by CD31 (green) and Endomucin (Emcn, red) high expression in 10-weeks old Dll4 iΔEC mutants and littermate control mice treated with PTH. Orientation of bone in the treated conditions is not affected. gp, growth plate. Scale bars, 300μm. Representative images from 3 repeated experiments. f, Maximum intensity projections of mouse tibial metaphysis immunostained for Endomucin (red), CD31 (green) and Itgb3 (white) to label blood capillaries and osteoclasts. PTH treatment promotes bone and blood vessel formation and does not inhibit osteoclasts in control mice. Dll4 iΔEC mutants show defective blood vessel growth. PTH treatment in Dll4 iΔEC mutants promoted local blood vessel growth. Scale bars, 50μm. Representative images from 3 repeated experiments.
Supplementary information
Supplementary Information
Supplementary Figures 1–7, Supplementary Table titles and legends.
Supplementary Table 1
Differentially expressed genes between VAO and BAO subsets.
Supplementary Table 2
Statistics source data.
Supplementary Table 3
Primer sequences.
Rights and permissions
About this article
Cite this article
Romeo, S.G., Alawi, K.M., Rodrigues, J. et al. Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation. Nat Cell Biol 21, 430–441 (2019). https://doi.org/10.1038/s41556-019-0304-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-019-0304-7
This article is cited by
-
Compensatory growth and recovery of cartilage cytoarchitecture after transient cell death in fetal mouse limbs
Nature Communications (2024)
-
Endothelial SMAD1/5 signaling couples angiogenesis to osteogenesis in juvenile bone
Communications Biology (2024)
-
Targeting strategies for bone diseases: signaling pathways and clinical studies
Signal Transduction and Targeted Therapy (2023)
-
NOX4 blockade suppresses titanium nanoparticle-induced bone destruction via activation of the Nrf2 signaling pathway
Journal of Nanobiotechnology (2022)
-
Osteoclast biology in the single-cell era
Inflammation and Regeneration (2022)