Abstract
Over their lifetime, long-term haematopoietic stem cells (HSC) are exposed to a variety of stress conditions that they must endure. Many stresses, such as infection/inflammation, reactive oxygen species, nutritional deprivation and hypoxia, activate unfolded protein response signalling, which induces either adaptive changes to resolve the stress or apoptosis to clear the damaged cell. Whether unfolded-protein-response signalling plays any role in HSC regulation remains to be established. Here, we report that the adaptive signalling of the unfolded protein response, IRE1α–XBP1, protects HSCs from endoplasmic reticulum stress-induced apoptosis. IRE1α knockout leads to reduced reconstitution of HSCs. Furthermore, we show that oncogenic N-RasG12D activates IRE1α–XBP1, through MEK–GSK3β, to promote HSC survival under endoplasmic reticulum stress. Inhibiting IRE1α–XBP1 abolished N-RasG12D-mediated survival under endoplasmic reticulum stress and diminished the competitive advantage of NrasG12D HSCs in transplant recipients. Our studies illuminate how the adaptive endoplasmic reticulum stress response is advantageous in sustaining self-renewal of HSCs and promoting pre-leukaemic clonal dominance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
References
Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 (2012).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Signer, R. A., Magee, J. A., Salic, A. & Morrison, S. J. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature 509, 49–54 (2014).
Wey, S., Luo, B. & Lee, A. S. Acute inducible ablation of GRP78 reveals its role in hematopoietic stem cell survival, lymphogenesis and regulation of stress signaling. PLoS ONE 7, e39047 (2012).
Miharada, K., Sigurdsson, V. & Karlsson, S. Dppa5 improves hematopoietic stem cell activity by reducing endoplasmic reticulum stress. Cell Rep. 7, 1381–1392 (2014).
Sigurdsson, V. et al. Bile acids protect expanding hematopoietic stem cells from unfolded protein stress in fetal liver. Cell Stem Cell 18, 522–532 (2016).
van Galen, P. et al. The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature 510, 268–272 (2014).
Kiel, M. J., Yilmaz, O. H., Iwashita, T., Terhorst, C. & Morrison, S. J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Oslowski, C. M. & Urano, F. in Methods in Enzymology Vol. 490 (ed. Conn, P. M.) 71–92 (Academic Press, Cambridge, 2011).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Iwawaki, T., Akai, R., Kohno, K. & Miura, M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 10, 98–102 (2004).
Osorio, F. et al. The unfolded-protein-response sensor IRE-1α regulates the function of CD8α+ dendritic cells. Nat. Immunol. 15, 248–257 (2014).
Ghosh, R. et al. Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 158, 534–548 (2014).
Iwawaki, T., Akai, R., Yamanaka, S. & Kohno, K. Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc. Natl Acad. Sci. USA 106, 16657–16662 (2009).
Raetz, C. R. & Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700 (2002).
Takizawa, H. et al. Pathogen-induced TLR4-TRIF innate immune signaling in hematopoietic stem cells promotes proliferation but reduces competitive fitness. Cell Stem Cell 21, 225–240.e5 (2017).
Zhang, H. et al. Sepsis induces hematopoietic stem cell exhaustion and myelosuppression through distinct contributions of TRIF and MYD88. Stem Cell Rep. 6, 940–956 (2016).
Li, S. et al. Lipopolysaccharide induces autophagic cell death through the PERK-dependent ranch of the unfolded protein response in human alveolar epithelial A549 cells. Cell. Physiol. Biochem. 36, 2403–2417 (2015).
Martinon, F., Chen, X., Lee, A. H. & Glimcher, L. H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11, 411–418 (2010).
Chen, Y. & Brandizzi, F. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 23, 547–555 (2013).
Notta, F. et al. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature 469, 362–367 (2011).
Rossi, D. J., Jamieson, C. H. & Weissman, I. L. Stems cells and the pathways to aging and cancer. Cell 132, 681–696 (2008).
Welch, J. S. et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 150, 264–278 (2012).
Pandolfi, A., Barreyro, L. & Steidl, U. Concise review: preleukemic stem cells: molecular biology and clinical implications of the precursors to leukemia stem cells. Stem Cells Transl. Med. 2, 143–150 (2013).
Li, Q. et al. Oncogenic Nras has bimodal effects on stem cells that sustainably increase competitiveness. Nature 504, 143–147 (2013).
Li, Q. et al. Hematopoiesis and leukemogenesis in mice expressing oncogenic Nras G12D from the endogenous locus. Blood 117, 2022–2032 (2011).
Cai, X. et al. Runx1 deficiency decreases ribosome biogenesis and confers stress resistance to hematopoietic stem and progenitor cells. Cell Stem Cell 17, 165–177 (2015).
Foudi, A. et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat. Biotechnol. 27, 84–90 (2009).
Ding, Q. et al. Erk associates with and primes GSK-3β for its inactivation resulting in upregulation of β-catenin. Mol. Cell 19, 159–170 (2005).
Song, L., De Sarno, P. & Jope, R. S. Central role of glycogen synthase kinase-3β in endoplasmic reticulum stress-induced caspase-3 activation. J. Biol. Chem. 277, 44701–44708 (2002).
Cui, Y. et al. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol. Cell. Biol. 24, 8037–8047 (2004).
Metcalf, D. et al. Gigantism in mice lacking suppressor of cytokine signalling-2. Nature 405, 1069–1073 (2000).
Ranatunga, S. et al. Synthesis of novel tricyclic chromenone-based inhibitors of IRE-1 RNase activity. J. Med. Chem. 57, 4289–4301 (2014).
Tang, C. H. et al. Inhibition of ER stress-associated IRE-1/XBP-1 pathway reduces leukemic cell survival. J. Clin. Invest. 124, 2585–2598 (2014).
Lee, A. H., Iwakoshi, N. N. & Glimcher, L. H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 23, 7448–7459 (2003).
Megias, J. et al. Direct Toll-like receptor-mediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages. Stem Cells 30, 1486–1495 (2012).
Esplin, B. L. et al. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J. Immunol. 186, 5367–5375 (2011).
Zismanov, V. et al. Phosphorylation of eIF2α is a translational control mechanism regulating muscle stem cell quiescence and self-renewal. Cell Stem Cell 18, 79–90 (2016).
Nguyen, H. G. et al. Development of a stress response therapy targeting aggressive prostate cancer. Sci. Trans. Med. 10, eaar2036 (2018).
Ma, Y. & Hendershot, L. M. The role of the unfolded protein response in tumour development: friend or foe? Nat. Rev. Cancer 4, 966–977 (2004).
Tay, K. H. et al. Sustained IRE1 and ATF6 signaling is important for survival of melanoma cells undergoing ER stress. Cell. Signal. 26, 287–294 (2014).
Moran-Crusio, K. et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 20, 11–24 (2011).
Acknowledgements
This work was supported by the University of Michigan Protein Folding Disease Initiative. Q.L. was supported by the NIH/NHLBI (grant no. 1R01HL132392), American Cancer Society (grant no. 125080-RSG-13-253-01-LIB), V Foundation for Cancer Research, Gabrielle’s Angel Foundation and Leukemia Research Foundation. J.D.V. is supported by the NIH (grant no. R01CA190860). Thanks to K. Haigis, T. Jacks, H. Hock, L. Hennighausen and M. Miura for providing LoxP-STOP-LoxP-NrasG12D, Col1α1-H2B-GFP; Rosa26-M2−rtTA, Stat5abfl and ERAI mice. We thank Z. Huang for the cloning vector for the ATF4 reporter constructs. We thank R. Signer (UCSD) for the critical reading and insightful feedback of our manuscript.
Author information
Authors and Affiliations
Contributions
L.L. performed most of the experiments. M.Z., G.N., K.B.Y. and X.J. performed some of the experiments with help from L.L. and Q.L. F.P., J.C. and X.C. contributed to the XBP1 splicing assay. T.I. contributed to the experiments with Ire1α knockout mice. J.D.V. contributed to the experiments with IRE1α inhibitors. L.L and Q.L. conceived the project, designed experiments, interpreted results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
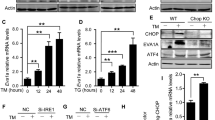
Supplementary Figure 1 ER stress activates UPR in murine long-term HSCs.
a, Gating strategy for FACS analysis and sorting of haematopoietic populations. b, Summary of qRT-PCR of UPR genes in purified CD48-LSKs treated with Tunicamycin or Thpasigargin for 1-24 hours. Levels were normalized to that of cells treated with vehicle for 1 hour. Bone marrow cells were pooled from 3 mice and FACS sorted for different haematopoietic populations. n=2 pools of samples were analyzed by qRT-PCR. c, Representative FACS plot showing ERAI signal in wild type HSCs treated with Thapsigargin (Tg) for 18 hours, together with IRE1α kinase inhibitor Kira6 (n=3 biological replicates in 3 independent experiments). d, Representative FACS plot showing ERAI signal in Myeloid (Mac-1+), B cell (B220+), T cell (CD3+) of wild type mice (3 biological replicates in 3 independent experiments). ERAI signal in HSCs isolated from mice without ERAI transgene (ERAI-) was used as negative control. e, Relative ERAI signal intensity (normalized to level in ERAI negative cells) in haematopoietic populations from Mx1-cre+; ERAI+; Ire1αfl/fl(fl/fl) and Mx1-cre-; ERAI+; Ire1αfl/fl (+/+) mice 2 weeks after pIpC injection (n=2 biological replicates in 2 independent experiments). f, ERAI signal intensity of HSCs freshly isolated from wild type mice (Fresh) or cultured for 12 hours (cultured) (n=3 biological replicates in 3 independent experiments). g, Representative FACS plot of haematopoietic stem and progenitor populations from wild type mice 24 hours after LPS injection (2 mg/kg body weight) (3 biological replicates in 3 independent experiments). h, FACS plot of Annexin V staining and ERAI signal in purified HSCs after 18 hours of Thapsigargin and proteasome inhibitor MG-132 treatment (2 biological replicates in 2 independent experiments). i, Representative FACS plot of Annexin V staining and ERAI signal in purified wild type HSC after 6-12 hours of Thapsigargin treatment (2 biological replicates in 2 independent experiments). Data represent mean±s.d. for all panels. Two-sided student t-test was used for statistical analysis.
Supplementary Figure 2 PERK activation as indicated by ATF4 reporter activity didn’t predict apoptosis in AML cell lines.
a, Lentiviral construct that expresses reporter gene DsRed express 2 under control of ATF4 uORFs that are activated by eIF2α (stress sensor, PE A4RP1.2), and its controls that express either constitutively active (PE A4RP3.2) or inactive (PE A4RP2.2) DsRed expression. b, Representative FACS plot of basal DsRed express 2 level in OCI-AML2 and OCI-AML3 cells transduced with these constructs. c-e, Representative FACS plots of DsRed Express 2 level in OCI-AML2 and OCI-AML3 cells transduced with stress sensor (c), constitutively inactive (d), or constitutively active (e) ATF4 reporter constructs and treated with vehicle (vehi), 5 mM Thapsigargin (Tg) or 10 μM Sal003 for 24 hours. Analysis was done on transduced cells (gated on ZsGreen positive cells). f, Representative FACS plots of Annexin V staining and ATF4 reporter signal in OCI-AML and OCI-AML3 cells treated with vehicle (vehi), 5 mM Thapsigargin (Tg) or 10 μM Sal003 for 24 hours (2 biological replicates in 2 independent experiments).
Supplementary Figure 3 Effects of IRE1α Knock-out on steady-state haematopoiesis.
a-g, Analysis of steady-state haematopoiesis in age- and sex-matched Mx1-cre+; IRE1αfl/fl(fl/fl) and Mx1-cre-; IRE1αfl/fl(+/+) mice at least 2 weeks post-pIpC injection (n=4 biological replicates in 4 independent experiments). a, Spleen weight. b, Cellularity in Bone marrow and spleen. c, Total numbers of haematopoietic stem and progenitor cells in the bone marrow and spleen. d, Frequency of haematopoietic stem and progenitor cells in bone marrow. e, Frequency of myeloid (Mac-1+), B (B220+) and T (CD3+) cells in the bone marrow. f, Frequency of haematopoietic stem and progenitor cells in the spleen. g, Frequency of myeloid (Mac-1+), B (B220+) and T (CD3+) cells in the spleen. Data represent mean±s.d. Two-sided student t-test was used for statistical analysis.
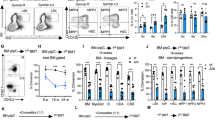
Supplementary Figure 4 N-RasG12D but not FLT3-ITD or Tet2 haploinsufficiency leads to HSC protection from ER stress-induced apoptosis.
a, Experimental scheme of the assay for results depicted in Figure 2a-e, b, Summary of the percentage of Annexin V positive cells in HSCs purified from 8-10 weeks old FLT3-ITD knock-in mice (FLT3-ITD/+) and sex-matched littermate control mice (+/+) and treated 18 hours of 0.6 μg/ml Tunicamycin(Tm) and 0.2 μM Thapsigargin (Tg) (n=3-5 biological replicates in 3 independent experiments). c, Summary of apoptosis analysis of HSCs from Mx1-cre-; Tet2fl/+ (+/+) or Mx1-cre+; Tet2fl/+ (fl/+) mice at least 2 weeks after 6 dose of pIpC injection, and treated with 18 hours of 0.6 μg/ml Tunicamycin(Tm) and 0.2 μM Thapsigargin (Tg) (n=3 biological replicates in 1 independent experiments). d, Experimental scheme of chimerism maintenance experiment for Figure 2f. 0.5 million bone marrow cells from Mx1-cre+; NrasG12D/+ (G12D/+) or control CD45.2 mice (2 weeks after pIpC injection) were transplanted with 0.5 million competitor (CD45.1) bone marrow cells into recipient mice. Six weeks after transplantation, transplant recipients were injected with 2 mg/kg LPS for 3 doses every other day and HSCs chimerism was analyzed 2-4 weeks after LPS injections. e, Experimental scheme of analysis in Col1α1-H2B-GFP; Rosa26-M2-rtTA mice. Mice were treated with doxycycline for 6 weeks to label HSCs with GFP, after which doxycycline water was removed to allow HSCs to dilute GFP signal upon cell division. GFPhigh and GFPlow HSCs (Gating strategy to separate GFPhigh and GFPlow HSCs is shown on the bottom right) were purified after 18 weeks off doxycycline and were treated with Tg or Tm for 18 hours. Data represent mean±s.d. for panels b and c. Two-sided student t-test was used for statistical analysis.
Supplementary Figure 5 MEK/ERK/GSK3β but not STAT5 is required for N-RasG12D mediated protection under ER stress.
a, Representative Western blot detecting phosphorylation of Serine 9 of GSK3β in CD48-LSK purified from Mx1-cre-; NrasG12D/+ (Control), or Mx1-cre; NrasG12D/+ (G12D/+), and then cultured for 10 minutes in the presence of 10 ng/ml SCF/TPO (3 biological replicates in 3 independent experiments). Original blots are shown in Supplementary Figure 7. b, Summary of the percentage of Annexin V positive HSC purified from Mx1-cre-; NrasG12D/+ (Control), or Mx1-cre+; NrasG12D/+ (G12D/+) mice that were treated with 0.2 μM Thapsigargin (Tg) together with 5 μM GSK3β inhibitor SB216763 (SB21) for 18 hours (n=3 independent experiments). c, Summary of the percentage of Annexin V positive HSCs from Mx1-cre-; NrasG12D/+ stat5a/bfl/+ or Mx1-cre-; NrasG12D/+ stat5a/b+/+ (Control), Mx1-cre+; NrasG12D/+ (G12D/+), Mx1-cre+; stat5a/bfl/+(STAT5), or Mx1-cre+; NrasG12D/+ stat5a/bfl/+ double mutant (DBM) mice after 0.6 μg/ml Tunicamycin (Tm) or 0.2 μM Thapsigargin(Tg) treatment for 18 hours (n=2 biological replicates in 2 independent experiments). d, Western blot detecting the level of SOCS2 and phosphorylation of STAT5 in CD48-LSK cells purified from SOCS2+/+ (+/+) and SOCS2+/- (+/-) mice. Original blots are shown in Supplementary Figure 7. (2-3 biological replicates in 1 independent experiments) e, Summary of the percentage of Annexin V positive CD48-LSK cells purified from 6-12 weeks old SOCS2+/+ (+/+) and SOCS2+/-(+/-) mice that were treated with 0.6 μg/ml Tunicamycin (Tm) or 0.2 μM Thapsigargin (Tg) for 18 hours (n=3 biological replicates for +/+, n=4 biological replicates for +/-, pooled from 2 independent experiments). f, Summary of the percentage of ERAI positive HSC purified from Mx1-cre-; ERAI+; NrasG12D/+ (Control), or Mx1-cre+; ERAI+; NrasG12D/+ (G12D/+) mice that were treated with 0.2 μM Thapsigargin (Tg) together with 100nM MEK inhibitor PD0325901 for 18 hours (n=5 biological replicates in 5 independent experiments). g, Summary of the percentage of ERAI positive HSC purified from ERAI+ mice treated with 0.2 μM Thapsigargin(Tg) together with 5 μM GSK3β inhibitor SB216763 (SB21) for 18 hours (n=3 biological replicates in 3 independent experiments). h, Schematic model showing that N-RasG12D-activated MEK/ERK signaling leads to phosphorylation and inhibition of GSK3β which results in activation of the adaptive IRE1α -XBP1 ER stress signaling to protect HSCs from ER stress-induced apoptosis. Data represent mean±s.d. . Two-sided student t-test was used for statistical analysis.
Supplementary Figure 6 IRE1α is hyper-activated in NrasG12D HSCs and is required for N-RasG12D mediated HSC protection, LSK and myeloid expansion.
a, Log ratio of the level of UPR target genes (measured by qRT-PCR) in Nras mutant HSCs relative to the level in control HSCs after tunicamycin (Tm) or thpasigargin (Tg) treatment (n=4 biological replicates in 2 independent experiments for Chop, Grp78, n=2 biological replicates in 2 independent experiments for Blos1,Pdgfrb,Hgsnat,Erdj4). b, Activation of IRE1α in different haematopoietic populations from Mx1-cre-; ERAI+; NrasG12D/+ (Control), or Mx1-cre+; ERAI+; NrasG12D/+ (G12D/+) mice injected with LPS was measured by mean induction of ERAI signal (Mean florescent intensity of ERAI in ERAI+ cells relative to ERAI- cells). (n=3 biological replicates in 3 independent experiments). c, Summary of the percentage of Annexin V positive HSC from Mx1-cre-; NrasG12D/+ (Control), Mx1-cre+; NrasG12D/+ (G12D/+) that were treated with 0.6 μg/ml tunicamycin (Tm) together with 20 μM IRE1α inhibitor BI-09 for 18 hours (n=2 biological replicates in 2 independent experiments). d-j, The steady state haematopoiesis in age- and sex-matched Mx1-cre-; NrasG12D/+ ;Ire1αfl/+(control) , Mx1-cre+; NrasG12D/+ ;Ire1α+/+(G12D/+) , Mx1-cre+; Nras+/+ ;Ire1αfl/+(Ire1α fl/+) ,Mx1-cre+; NrasG12D/+ ;Ire1αfl/+(DBM) mice was analyzed 2 weeks post-pIpC injection. d Spleen weight. n=9 (control, G12D/+ and DBM), and 3 (IRE1α fl/+) biological replicates in 6 independent experiments, e, Cellularity in Bone marrow and spleen. .n=7 (control, G12D/+ and DBM), and 2 (IRE1α fl/+) biological replicates in 6 independent experiments, f, Total numbers of haematopoietic stem and progenitor cells in bone marrow and spleen. .n=6 (control, G12D/+ and DBM), and 2 (IRE1α fl/+) biological replicates in 6 independent experiments,. g-h, Frequency of haematopoietic stem and progenitor cells in bone marrow (g) and spleen (h). . n=6 (control, G12D/+ and DBM), and 3 (IRE1α fl/+) biological replicates in 6 independent experiments,. I-j, Frequency of lineage cells in bone marrow (i) and spleen (j). . n=8 (control, G12D/+ and DBM), and 3 (IRE1α fl/+) biological replicates in 6 independent experiments, Data represent mean±s.d. Two-sided student t-test was used for statistical analysis.
Supplementary Figure 7 Original images of Western blots and DNA gel.
While boxes indicate the images used for figures. Because of the small number of HSCs (5,000-10,000 cells/mouse) or CD48-LSK (contains HSCs and MPPs; 20,000-30,000 cells/mouse), to ensure adequate protein amount, the entire protein lysates from one set of FACS-sorted samples were used for one SDS-PACE gel. The Western membranes were then sectioned into 3-4 strips based on molecular weight of the standard protein ladder, to allow simultaneous probing for multiple proteins on using the same protein samples. Images for Supplementary Figure 5d represents a Western membrane that was cut into 4 strips, each probed for different antibody. The strips were then aligned together for autoradiography. Three different exposure times were included to show the detection of different proteins.
Supplementary information
Supplementary Information
Supplementary Figures 1–7 and Supplementary Table legends.
Supplementary Table 1
List of all antibodies used for experiments.
Supplementary Table 2
Statistic source data.
Rights and permissions
About this article
Cite this article
Liu, L., Zhao, M., Jin, X. et al. Adaptive endoplasmic reticulum stress signalling via IRE1α–XBP1 preserves self-renewal of haematopoietic and pre-leukaemic stem cells. Nat Cell Biol 21, 328–337 (2019). https://doi.org/10.1038/s41556-019-0285-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-019-0285-6
This article is cited by
-
C/EBPα-p30 confers AML cell susceptibility to the terminal unfolded protein response and resistance to Venetoclax by activating DDIT3 transcription
Journal of Experimental & Clinical Cancer Research (2024)
-
MITOL deficiency triggers hematopoietic stem cell apoptosis via ER stress response
The EMBO Journal (2024)
-
Experimental periodontitis induced hypoadiponectinemia by IRE1α-mediated endoplasmic reticulum stress in adipocytes
BMC Oral Health (2023)
-
EVA1A regulates hematopoietic stem cell regeneration via ER-mitochondria mediated apoptosis
Cell Death & Disease (2023)
-
A novel HIF-2α targeted inhibitor suppresses hypoxia-induced breast cancer stemness via SOD2-mtROS-PDI/GPR78-UPRER axis
Cell Death & Differentiation (2022)