Abstract
Although the aetiology of amyotrophic lateral sclerosis (ALS) remains poorly understood, impaired proteostasis is a common feature of different forms of ALS. Mutations in genes encoding ubiquilins, UBQLN2 and UBQLN4, cause familial ALS. The role of ubiquilins in proteasomal degradation is well established, but their role in autophagy–lysosomal clearance is poorly defined. Here, we describe a crosstalk between endoplasmic reticulum stress, mTOR signalling and autophagic flux in Drosophila and mammalian cells lacking ubiquilins. We found that loss of ubiquilins leads to endoplasmic reticulum stress, impairs mTORC1 activity, promotes autophagy and causes the demise of neurons. We show that ubiquilin mutants display defective autophagic flux due to reduced lysosome acidification. Ubiquilins are required to maintain proper levels of the V0a/V100 subunit of the vacuolar H+-ATPase and lysosomal pH. Feeding flies acidic nanoparticles alleviates defective autophagic flux in ubiquilin mutants. Hence, our studies reveal a conserved role for ubiquilins as regulators of autophagy by controlling vacuolar H+-ATPase activity and mTOR signalling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Code availability
There is no custom code used in this study.
Data availability
The MS proteomics data can be accessed from the ProteomeXchange Consortium via the MassIVE repository (MSV000083259) under accession code PXD012104. The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Information files. Source data for Figs. 1c,f, 2c–e, 3b–f, 4a,d–f, 5e, 6a,c,d,g, 7a–d, and Supplementary Figs. 1f, 2a,d,e, 3a–c, 4b,c,g,j, 5a,e,f, 7b,c, and 8b–d have been provided as Supplementary Table 9. All other data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Edens, B. M. et al. A novel ALS-associated variant in UBQLN4 regulates motor axon morphogenesis. eLife 6, 3797 (2017).
Deng, H.-X. et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 477, 211–215 (2011).
Ko, H. S., Uehara, T., Tsuruma, K. & Nomura, Y. Ubiquilin interacts with ubiquitylated proteins and proteasome through its ubiquitin-associated and ubiquitin-like domains. FEBS Lett. 566, 110–114 (2004).
Kleijnen, M. F. et al. The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome. Mol. Cell 6, 409–419 (2000).
Lim, P. J. et al. Ubiquilin and p97/VCP bind erasin, forming a complex involved in ERAD. J. Cell Biol. 187, 201–217 (2009).
N’Diaye, E.-N. et al. PLIC proteins or ubiquilins regulate autophagy-dependent cell survival during nutrient starvation. EMBO Rep. 10, 173–179 (2009).
Rothenberg, C. et al. Ubiquilin functions in autophagy and is degraded by chaperone-mediated autophagy. Hum. Mol. Genet. 19, 3219–3232 (2010).
Biggins, S., Ivanovska, I. & Rose, M. D. Yeast ubiquitin-like genes are involved in duplication of the microtubule organizing center. J. Cell Biol. 133, 1331–1346 (1996).
Itakura, E. et al. Ubiquilins chaperone and triage mitochondrial membrane proteins for degradation. Mol. Cell 63, 21–33 (2016).
Hjerpe, R. et al. UBQLN2 mediates autophagy-independent protein aggregate clearance by the proteasome. Cell 166, 935–949 (2016).
Yamamoto, S. et al. A Drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases. Cell 159, 200–214 (2014).
Haelterman, N. A. et al. Large-scale identification of chemically induced mutations in Drosophila melanogaster. Genome Res. 24, 1707–1718 (2014).
Sarov, M. et al. A genome-wide resource for the analysis of protein localisation in Drosophila. eLife 5, e12068 (2016).
Venken, K. J. T. et al. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nat. Methods 6, 431–434 (2009).
Li-Kroeger, D. et al. An expanded toolkit for gene tagging based on MiMIC and scarless CRISPR tagging in Drosophila. eLife 7, e1002472 (2018).
Chou, T. B., Noll, E. & Perrimon, N. Autosomal P[ovoD1] dominant female-sterile insertions in Drosophila and their use in generating germ-line chimeras. Development 119, 1359–1369 (1993).
LaJeunesse, D. R. et al. Three new Drosophila markers of intracellular membranes. Biotechniques 36, 784–790 (2004).
Moustaqim-Barrette, A. et al. The amyotrophic lateral sclerosis 8 protein, VAP, is required for ER protein quality control. Hum. Mol. Genet. 23, 1975–1989 (2014).
Hetz, C. & Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 13, 477–491 (2017).
Ogata, M. et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 26, 9220–9231 (2006).
Rashid, H.-O., Yadav, R. K., Kim, H.-R. & Chae, H.-J. ER stress: autophagy induction, inhibition and selection. Autophagy 11, 1956–1977 (2015).
Høyer-Hansen, M. & Jäättelä, M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 14, 1576–1582 (2007).
Senft, D. & Ronai, Z. A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 40, 141–148 (2015).
Qin, L., Wang, Z., Tao, L. & Wang, Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy 6, 239–247 (2014).
Kamada, Y. et al. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J. Cell Biol. 150, 1507–1513 (2000).
Matsuura, A., Tsukada, M., Wada, Y. & Ohsumi, Y. Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene 192, 245–250 (1997).
Le Ber, I. et al. SQSTM1 mutations in French patients with frontotemporal dementia or frontotemporal dementia with amyotrophic lateral sclerosis. JAMA Neurol. 70, 1403–1410 (2013).
Komatsu, M. et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131, 1149–1163 (2007).
Denton, D. et al. Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Curr. Biol. 19, 1741–1746 (2009).
Kimura, S., Noda, T. & Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3, 452–460 (2007).
Mauvezin, C., Nagy, P., Juhász, G. & Neufeld, T. P. Autophagosome–lysosome fusion is independent of V-ATPase-mediated acidification. Nat. Commun. 6, 7007 (2015).
Neufeld, T. P. & Baehrecke, E. H. Eating on the fly: function and regulation of autophagy during cell growth, survival and death in Drosophila. Autophagy 4, 557–562 (2008).
Scott, R. C., Schuldiner, O. & Neufeld, T. P. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell 7, 167–178 (2004).
Kinouchi, K. et al. The role of individual domains and the significance of shedding of ATP6AP2/(pro)renin receptor in vacuolar H(+)-ATPase biogenesis. PLoS ONE 8, e78603 (2013).
Bareford, L. M. & Swaan, P. W. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 59, 748–758 (2007).
Baltazar, G. C. et al. Acidic nanoparticles are trafficked to lysosomes and restore an acidic lysosomal pH and degradative function to compromised ARPE-19 cells. PLoS ONE 7, e49635 (2012).
Zhang, Y., Krieger, V. & Hensel, M. Application of fluorescent nanoparticles to study remodeling of the endo-lysosomal system by intracellular bacteria. J. Vis. Exp. 95, e52058 (2015).
Bourdenx, M. et al. Nanoparticles restore lysosomal acidification defects: implications for Parkinson and other lysosomal-related diseases. Autophagy 12, 472–483 (2016).
Lee, J.-H. et al. Presenilin 1 maintains lysosomal Ca2+ homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep. 12, 1430–1444 (2015).
Kim, T.-Y., Kim, E., Yoon, S. K. & Yoon, J.-B. Herp enhances ER-associated protein degradation by recruiting ubiquilins. Biochem. Biophys. Res. Commun. 369, 741–746 (2008).
Mendes, C. S. et al. ER stress protects from retinal degeneration. EMBO J. 28, 1296–1307 (2009).
Wang, L., Popko, B., Tixier, E. & Roos, R. P. Guanabenz, which enhances the unfolded protein response, ameliorates mutant SOD1-induced amyotrophic lateral sclerosis. Neurobiol. Dis. 71, 317–324 (2014).
Wang, L., Popko, B. & Roos, R. P. An enhanced integrated stress response ameliorates mutant SOD1-induced ALS. Hum. Mol. Genet. 23, 2629–2638 (2014).
Mao, D. et al. VAMP associated proteins are required for autophagic and lysosomal degradation by promoting a PtdIns4P mediated endosomal pathway. Autophagy https://doi.org/10.1080/15548627.2019.1580103 (2019).
Pandey, U. B. et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447, 859–863 (2007).
Appenzeller-Herzog, C. & Hall, M. N. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol. 22, 274–282 (2012).
Sancak, Y. et al. Ragulator–Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290–303 (2010).
Zoncu, R. et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334, 678–683 (2011).
Arias, E. et al. Lysosomal mTORC2/PHLPP1/Akt regulate chaperone-mediated autophagy. Mol. Cell 59, 270–284 (2015).
Chen, C.-H. et al. ER stress inhibits mTORC2 and Akt signaling through GSK-3β-mediated phosphorylation of rictor. Sci. Signal. 4, ra10–ra10 (2011).
Williamson, W. R., Wang, D., Haberman, A. S. & Hiesinger, P. R. A dual function of V0-ATPase a1 provides an endolysosomal degradation mechanism in Drosophila melanogaster photoreceptors. J. Cell Biol. 189, 885–899 (2010).
Haberman, A. et al. The synaptic vesicle SNARE neuronal Synaptobrevin promotes endolysosomal degradation and prevents neurodegeneration. J. Cell Biol. 196, 261–276 (2012).
Hermle, T., Guida, M. C., Beck, S., Helmstädter, S. & Simons, M. Drosophila ATP6AP2/VhaPRR functions both as a novel planar cell polarity core protein and a regulator of endosomal trafficking. EMBO J. 32, 245–259 (2013).
Dubos, A. et al. Conditional depletion of intellectual disability and parkinsonism candidate gene ATP6AP2 in fly and mouse induces cognitive impairment and neurodegeneration. Hum. Mol. Genet. 24, 6736–6755 (2015).
Jiang, S. et al. Oral administration and selective uptake of polymeric nanoparticles in Drosophila larvae as an in vivo model. ACS Biomat. Sci. Eng. 1, 1077–1084 (2015).
Chung, H. J., Kim, H. K., Yoon, J. J. & Park, T. G. Heparin immobilized porous PLGA microspheres for angiogenic growth factor delivery. Pharm. Res. 23, 1835–1841 (2006).
Verstreken, P. et al. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron 40, 733–748 (2003).
Chouhan, A. K. et al. Uncoupling neuronal death and dysfunction in Drosophila models of neurodegenerative disease. Acta Neuropathol. Commun. 4, 62 (2016).
Yoon, W. H. et al. Loss of nardilysin, a mitochondrial co-chaperone for α-ketoglutarate dehydrogenase, promotes mtorc1 activation and neurodegeneration. Neuron 93, 115–131 (2017).
Hiesinger, P. R. et al. The v-ATPase V0 Subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell 121, 607–620 (2005).
Aniento, F., Roche, E., Cuervo, A. M. & Knecht, E. Uptake and degradation of glyceraldehyde-3-phosphate dehydrogenase by rat liver lysosomes. J. Biol. Chem. 268, 10463–10470 (1993).
di Ronza, A. et al. CLN8 is an endoplasmic reticulum cargo receptor that regulates lysosome biogenesis. Nat. Cell Biol. 17, 701 (2018).
Acknowledgements
The authors thank past and current members of the Bellen Lab for technical assistance and discussions, and thank T. Orand, W. H. Yoon, A. Di Ronza, H. V. Bhavana, H. Pan and L. Duraine for technical support. Thanks are also given to the following: S. N. Jaiswal for helpful discussions and comments; K. Schulze, M. Campbell and B. Ugur for their comments on the manuscript; the BDSC (NIH P40OD018537) for Drosophila stocks; S. Y. Jung, A. Jain and the BCM Pathway Discovery Proteomics Core (CPRIT RP120092 and P30CA125123). Microscopy was supported in part by the Baylor College of Medicine IDDRC Neurovisualization Core (U54HD083092) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. E.W. received support from a Ruth L. Kirschstein Fellowship from the National Institute of Dental and Craniofacial Research (F31 DE027586). H.J.B. is supported by the Robert A. and Renee E. Belfer Family Foundation, the Huffington Foundation, Target ALS and the NIH Office of the Director (R24OD022005). H.J.B is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
M.S. conceived the project, designed and performed the majority of the experiments, and analysed the data. M.S. and Z.Z. performed the TEM and histology experiments. E.W. and A.G.M. synthesized the aNPs. M.S., G.L. and D.M. performed the cell culture experiments. M.S. and H.J.B. wrote the manuscript. H.J.B. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Ubiquilin is a broadly expressed essential gene.
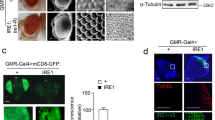
(a) Confocal microscopy images of larval brain (with ventral nerve cord (VNC) and imaginal discs), body wall muscle (6/7 of segment A3), salivary gland, and gut of third instar larva expressing GFP-tagged genomic Ubqn transgene (Ubqn-GFP construct). Scale bars, 50 μm. (b) Western blot for Ubqn with protein lysates from third instar larvae of actin(act)-GAL4/+, act-GAL4>Ubqn IR, act-GAL4>Ubqn cDNA, ubqn1; GR, and ubqn1. (c) Western blot for Ubqn with protein lysates from third instar larvae of iso (Ctrl) and ywing2+Δubqn. (d) Photographs of control (iso) embryo and ubqn1 embryo (maternal contribution removed by homozygous germ line clones generated in y w ubqn1 FRT19A/ovoD1 FRT19A females). (e) Ventral axis photographs of late P4 stage pre-pupa of iso (control) and ubqn1. Arrows indicate the extension of legs and asterisk indicates morphologically defective head. Scale bars, 200 μm. (f) H&E staining in head trans-sections of control (iso) and ubqn1 pre-pupae with quantification of fat content per head section. n = 4 biologically independent flies. Mean ± s.e.m. **p =0.0084. Scale bars, 100 μm. For all panels except S1c and S1f, three independent experiments were performed with similar results. For S1c and S1f, two independent experiments were performed. All statistics were determined by two-sided Student’s t-test. Statistics source data for Supplementary Fig. 1f can be found in Supplementary Table 9.
Supplementary Figure 2 Loss of Ubqn leads to neurodegeneration.
(a) TEM image of 1day- old and 15 day-old PR of ey-FLP clones of ubqn1; GR with quantification of PR number per ommatidium of ey-FLP clones of ubqn1; GR and ubqn1. n = 44 (ubqn1; GR D1), n = 37 (ubqn1 D1), n = 41 (ubqn1; GR D15), and n = 40 (ubqn1 D15) ommatidia from 3 animals. ns, not significant. Scale bars, 4 μm. (b) TEM image of 15 day-old PR of ey-FLP clones of ubqn1; GR and ubqn1 with degenerative features. Scale bars, 2 μm. (c) TEM images of 30 day-old PRs and synaptic terminals of ey-FLP clones of ubqn1; GR and ubqn1, revealing mitochondria accumulation. Flies are raised in 12h light/12h dark cycle. Scale bars, 1 μm. (d) TEM images of 30 day-old synaptic terminals of ey-FLP clones of ubqn1; GR and ubqn1 with quantification of average synapse area. Individual synaptic terminals are highlighted in pink. n = 30 (ubqn1; GR D1), n = 26 (ubqn1 D1), n = 37 (ubqn1; GR D30 and ubqn1 D30) terminals from 3 animals. Mean ± s.e.m. ns, not significant; ****p< 0.0001. Scale bars, 2 μm. (e) ERG traces from 45 day-old ey-FLP clones of iso (control) and ubqn1 raised in 24h dark cycle (DD) with quantification of ERG amplitudes. n = 4 flies. Mean ± s.e.m. ****p< 0.0001. (f) Phalloidin staining of 30 day-old ey-FLP clones of iso (control) and ubqn1 raised in 24h dark cycle (DD). Scale bars, 5 μm. For all panels, three independent experiments were performed with similar results. All statistics were determined by two-sided Student’s t-test. Statistics source data for Supplementary Fig. 2a, d, e can be found in Supplementary Table 9.
Supplementary Figure 3 Loss of Ubqn leads to aberrant ER and proteostasis defects.
(a) Confocal imaging of ubiquitously expressing EYFP-ER marker in ubqn1 clones (marked white) in third instar larval salivary glands with quantification of normalized EYFP density in ubqn1 clones and the surrounding control cells. Scale bars, 10 μm. n = 5 biologically independent samples. Mean ± s.e.m. ***p = 0.0003. (b) Immunofluorescence staining for Chaoptin and ELAV (labelling neuronal nuclei) in adult brain cortex of 2–3 days-old control flies (N-syb>Luc IR) and flies with pan-neuronal knockdown of Ubqn (N-syb>Ubqn IR) and quantification of neurons that accumulate Chaoptin. Scale bars, 20 μm. n = 7 (N-syb>LucIR), n = 6 (N-syb>ubqnIR) biologically independent samples. Mean ± s.e.m. **p = 0.0017. (c) Western blot for BiP (Hsc3) with fat body protein lysates form third instar iso (control) and ubqn1 with quantification of relative BiP/actin levels. n=4 biologically independent samples. Mean ± s.e.m. **p = 0.0025. (d-e) Xbp1-EGFP marker allows EGFP expression only after IRE1α-mediated splicing of Xbp1 mRNA. (d) Western blot for GFP with third instar larval lysates of iso (control), ubqn1, and ubqn1; GR expressing Xbp1-EGFP. (e) Immunofluorescence staining for GFP from third instar larval muscle (segment A3 Muscle 7) of iso (control) and ubqn1 expressing Xbp1-EGFP. Scale bars, 10 μm. (f) Western blot for Phospho-eIF2α (S51) and total eIF2α with protein lysates from third instar larvae of iso (control) and Δubqn. For all panels except S3d-f, three independent experiments were performed with similar results. For panels S3d-f, two independent experiments were performed with similar results. All statistics were determined by two-sided Student’s t-test. Statistics source data for Supplementary Fig. 3a–c can be found in Supplementary Table 9.
Supplementary Figure 4 Loss of Ubqn impairs autophagy-lysosomal degradation.
(a) ubqn1 clone in fat body of early third instar larvae expressing UAS-Atg8a-GFP with Cg-GAL4 driver. Scale bar, 10 μm. (b) TEM images of the synaptic terminals of 15-day old ey-FLP clones of ubqn1; GR and ubqn1 with quantification of normalized autophagosome number. Individual synaptic terminals are highlighted in pink and arrowheads show autophagosomes. n = 3 flies. Mean ± s.e.m. **p = 0.002. Scale bars, 1 μm. (c) qRT-PCR quantification showing ref2(p)/p62 expression in ubqn1 third instar larvae compared to iso (y w FRT19A) larvae normalized against housekeeping gene (GAPDH). n = 3 independent biological samples and 3 PCR replicates for each biological sample. Mean ± s.e.m. ns, not significant. (d) Western blots for p62 and Atg8 with early third instar larval brain protein lysates of iso (Ctrl) and ubqn1 that are fed or 4h starved in 20% sucrose. (e) Western blot for p62 with fat body protein lysates from third instar larvae of ubqn1; GR (control) and ubqn1 that are starved for 1h, 2h, or 4h in 20% sucrose. (f) H&E staining in trans-sections of control (iso) and ubqn1 pre-pupae. Larval midgut is pseudo-coloured in yellow in insets. Scale bars, 200 μm. (g) 4 hour starved early third instar larval fat body of y w (control) and ubqn1 expressing UAS-GFP-mCherry-Atg8a with Cg-GAL4 driver and quantification of only mCherry expressing and GFP+mCherry expressing punctae/section (135 μm2) of starved larvae. n = 10 (control), n = 9 (ubqn1) biologically independent samples. Mean ± s.e.m. ***p = 0.0003, ****p< 0.0001. Scale bars, 10 μm. (h) ubqn1 clone (-/-) in fat body of fed and starved second instar larvae expressing UAS-LAMP1-GFP with Cg-GAL4 driver. Scale bars, 10 μm. (i) Live imaging for LysoTracker (LT) dye in fed and starved second instar larval fat body with ubqn1 clones. Scale bars, 10 μm. (j) qRT-PCR quantification showing expressions of V100-2, VhaSFD, Vha16-1, Vha68-2, VhaAC45, Atg8a, V100-1, and VhaM8.9 in ubqn1 third instar larvae compared to iso (control) larvae normalized against housekeeping gene (GAPDH). n = 3 independent biological samples with 3 PCR replicates for biological samples of: VhaSFD, Vha16-1, Vha68-2, VhaAC45, Atg8a, and 6 PCR replicates for biological samples of: V100-2, V100-1, VhaM8.9. Mean ± s.e.m. ns, not significant. *p = 0.00138, ****p< 0.0001. For all panels except S4d-f, three independent experiments were performed with similar results. For S4d-f, two independent experiments were performed with similar results. All statistics were determined by two-sided Student’s t-test. Statistics source data for Supplementary Fig. 4b, c, g and j can be found in Supplementary Table 9.
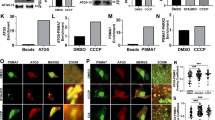
Supplementary Figure 5 Ubiquilin genetically interacts with v-ATPase subunits.
(a) Western blots for Vha55 (V1B2 in mammals) and genomically GFP tagged VhaSFD with protein lysates from third instar larvae of control (iso), ubqn1, y w;VhaSFD-GFP, and ubqn1;VhaSFD-GFP and quantification of relative protein levels. n = 4 biologically independent samples. Mean ± s.e.m. ns, not significant. (b) Western blot for V100 with protein lysates from third instar larvae of ubqn1; GR (control), ubqn1, ubqn1; v100/+, and ubqn1; vhaM8.9/+. (c) Western blot for V100 in homogenate (H) and lysosome (L) fractions from third instar larvae of iso (control) and ubqn1. Western blots for Vha55 (a V1 subunit) and Tubulin indicate lysosome enrichment upon subcellular fractionation. (d) Western blots for V100, p62, P-eIF2α, and eIF2α with fat body protein lysates from third instar larvae of w; Cg-GAL4/+, w; Cg-GAL4/UAS-V100 IR, and w; Cg-GAL4/UAS-V100. (e) Live imaging for LysoTracker (LT) dye in third instar larval fat body of w; Cg-GAL4/+, w; Cg-GAL4/UAS-V100 IR, and w; Cg-GAL4/UAS-V100 with quantification of relative LT intensity. n = 7 biologically independent samples. Mean ± s.e.m. ns, not significant; ***p = 0.0002. (f) Confocal imaging of EYFP-ER marker in ubqn1 clones (marked white) in third instar larval salivary glands of wild type (v100+/+) or heterozygous (v1003/+) animals with quantification of EYFP ratio in ubqn1 clones and the surrounding cells. Scale bars, 10 μm. n = 4 biologically independent samples. Mean ± s.e.m. ns, not significant. (g) Western blots for P-eIF2α and eIF2α with protein lysates from third instar larvae of control (iso), ubqn1, and ubqn1; v100/+ with quantification of P-eIF2α to total eIF2α ratio. n = 4 biologically independent samples. Mean ± s.e.m. ns, not significant. (h) Western blots for P-S6K and S6K with fat body protein lysates from third instar larvae of control (iso), ubqn1, and ubqn1; v100/+. For all panels except S5f and S5h, three independent experiments were performed with similar results. For S5f and S5h, two independent experiments were performed with similar results. All statistics were determined by two-sided Student’s t-test. Statistics source data for Supplementary Fig. 5a, e, f and g can be found in Supplementary Table 9.
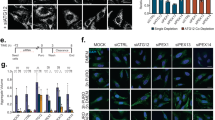
Supplementary Figure 6 Acidic nanoparticle feeding in flies.
(a) Chemical reaction showing hydrolysis of Poly(lactic-co-glycolic acid) (PLGA). (b) Scanning electron microscopy images showing the surface morphology of PLGA, BSA-PLGA, and BSA-NR-PLGA nanoparticles after coating with 10 nm of gold. Imaging was performed once. Scale bars, 0.5 μm. (c) Western blots for P-eIF2α, eIF2α, P-S6K, and S6K with fat body protein lysates from third instar larvae of control (iso) and ubqn1 either fed by standard food (0 mg/mL) or standard food with 3mg/mL aNPs for 3 hours. Two independent experiments were performed with similar results.
Supplementary Figure 7 Ubiquilin’s role in regulation of lysosome acidification is evolutionarily conserved.
(a) Western blot for UBQLN1, UBQLN2, and UBQLN4 with Daoy whole cell lysates of siCtrl and UBQLN1+2+4 knockdown (siUBQLNs) cells. Asterisk indicates nonspecific band in UBQLN4 blot. (b) Western blot for Phospho-eIF2α (S51) and total eIF2α with Daoy whole cell lysates of siCtrl and UBQLN1+2+4 knockdown (siUBQLNs) cells and quantification of relative P-eIF2α/eIF2α ratio. n = 4 biologically independent samples. Mean ± s.e.m. ***p = 0.0005. (c) Immunofluorescence staining with LC3 and DAPI in siCtrl and siUBQLNs Daoy cells with or without 4 hour Bafilomycin A1 treatment and quantification of normalized mean LC3 fluorescence intensity per cell. n = 5 images (~40 cells). Mean ± s.e.m. **p = 0.0036, ****p< 0.0001. Scale bars, 10 μm. (d) Western blots for UBQLN1, UBQLN2, and UBQLN4 with HEK293 whole cell lysates of control and UBQLN1+2+4 triple knockout (TKO) cells. Asterisk indicates nonspecific band in UBQLN4 blot. (e) Western blots for p62 and LC3 with HEK293 whole cell lysates of control and TKO cells with or without 4 hour Bafilomycin A1 treatment. (f) Western blots for LAMP1, LAMP2, and v-ATPase subunits: V0a1, V1B2, V1D1 V1H, and M8.9 with HEK293 whole cell lysates of control and TKO cells. fl: full length, fg: fragmented. (g) Immunoprecipitation of UBQLN1-FLAG or UBQLN2-FLAG with FLAG beads shows that UBQLN1 and UBQLN2 interact with endogenous V0a1 in HEK293T cells transfected with UBQLN1-FLAG or UBQLN2-FLAG.For all panels except for S7g, three independent experiments were performed with similar results. For S7g, two independent experiments were performed with similar results. All statistics were determined by two-sided Student’s t-test. Statistics source data for Supplementary Fig. 7b, c can be found in Supplementary Table 9.
Supplementary Figure 8 UBQLN4 depletion impairs lysosomal degradation.
(a) Western blot for UBQLN1, UBQLN2, and UBQLN4 with Daoy whole cell lysates of siCtrl, siUBQLN1, siUBQLN2, siUBQLN4, and siUBQLN1+2+4 knockdown cells. Asterisks indicate nonspecific bands in UBQLN4 blot. (b) Western blot for Phospho-eIF2α (S51) and total eIF2α with Daoy whole cell lysates of siCtrl, siUBQLN1, siUBQLN2, siUBQLN4, and siUBQLN1+2+4 knockdown cells with quantification of relative P-eIF2α/eIF2α ratio. n = 3 biologically independent samples. Mean ± s.e.m. **p = 0.0011 (siUBQLN1), **p = 0.0089 (siUBQLN1+2+4). (c) Western blot for p62 with Daoy whole cell lysates of siCtrl, siUBQLN1, siUBQLN2, siUBQLN4, and siUBQLN1+2+4 knockdown cells with or without 4 hour Bafilomycin A1(BafA1) treatment and quantification of relative p62/GAPDH levels. n = 3 biologically independent samples. Mean ± s.e.m. ns, not significant; *p = 0.0148, **p = 0.0022 (DMSO-siUBQLN4), **p = 0.0026 (DMSO-siUBQLN1+2+4), **p = 0.0019 (BafA1-siUBQLN1+2+4). (d) Live imaging for LysoTracker (LT) in siCtrl, siUBQLN1, siUBQLN2, siUBQLN4, and siUBQLN1+2+4 knockdown Daoy cells with quantification of normalized mean LT fluorescence intensity per cell. n = 5 images (~20 cells). Mean ± s.e.m. ns, not significant; **p = 0.0039, ***p = 0.0005 (siUBQLN4), ***p = 0.0002 (siUBQLN1+2+4). Scale bars, 10 μm. For all panels except S8d, three independent experiments were performed with similar results. For panel S8d, two independent experiments were performed with similar results. All statistics were determined by two-sided Student’s t-test. Statistics source data for Supplementary Fig. 8b–d can be found in Supplementary Table 9.
Supplementary Figure 9
Unprocessed images of all gels and blots.
Supplementary information
Supplementary Information
Supplementary Figures 1–9 and Supplementary Table legends.
Supplementary Table 1
Ubqn is the Drosophila homologue of hUBQLNs.
Supplementary Table 2
Suppression of ubqn1 lethality by genomic and cDNA constructs.
Supplementary Table 3
Protein hits identified as Ubqn interactors in all three IP/MS experiments.
Supplementary Table 4
v-ATPase subunits identified by IP/MS.
Supplementary Table 5
Genetic interaction between Ubqn and v-ATPase subunits.
Supplementary Table 6
Characteristics of acidic nanoparticles (aNPs).
Supplementary Table 7
Primary antibodies used in the study.
Supplementary Table 8
Primers used for the qRT-PCR.
Supplementary Table 9
Statistics source data.
Rights and permissions
About this article
Cite this article
Şentürk, M., Lin, G., Zuo, Z. et al. Ubiquilins regulate autophagic flux through mTOR signalling and lysosomal acidification. Nat Cell Biol 21, 384–396 (2019). https://doi.org/10.1038/s41556-019-0281-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-019-0281-x
This article is cited by
-
Defective lysosomal acidification: a new prognostic marker and therapeutic target for neurodegenerative diseases
Translational Neurodegeneration (2023)
-
A neuroprotective role of Ufmylation through Atg9 in the aging brain of Drosophila
Cellular and Molecular Life Sciences (2023)
-
3BDO Alleviates Seizures and Improves Cognitive Function by Regulating Autophagy in Pentylenetetrazol (PTZ)-Kindled Epileptic Mice Model
Neurochemical Research (2022)
-
Carrying Excess Baggage Can Slowdown Life: Protein Clearance Machineries That Go Awry During Aging and the Relevance of Maintaining Them
Molecular Neurobiology (2022)
-
Autophagy Dysfunction in ALS: from Transport to Protein Degradation
Journal of Molecular Neuroscience (2022)