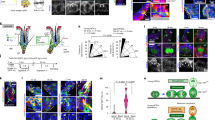
Abstract
Tissue homeostasis is sustained by stem cell self-renewal and differentiation. How stem cells coordinately differentiate into multiple cell types is largely unclear. Recent studies underline the heterogeneity among stem cells or common progenitors, suggesting that coordination occurs at the stem cell/progenitor level1,2,3,4. Here, by tracking and manipulating the same stem cells and their progeny at the single-cell level in live mice, we uncover an unanticipated flexibility of homeostatic stem cell differentiation in hair follicles. Although stem cells have been shown to be flexible upon injury, we demonstrate that hair germ stem cells at the single-cell level can flexibly establish all of the differentiation lineages even in uninjured conditions. Furthermore, stem cell-derived hair progenitors in the structure called matrix, previously thought to be unipotent, flexibly change differentiation outcomes as a consequence of unexpected dynamic relocation. Finally, the flexible cell fate determination mechanism maintains normal differentiation and tissue architecture against an ectopic differentiation stimulus induced by Wnt activation. This work provides a model of continual fate channelling and late commitment of stem cells to achieve coordinated differentiation and robust tissue architecture.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Statistics source data for Fig. 3c and Supplementary Fig. 1b have been provided as Supplementary Table 1. Additional images for Fig. 5 have been deposited at Figshare: https://doi.org/10.6084/m9.figshare.7170422. All of the data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Paul, F. et al. Transcriptional heterogeneity and lineage commitment in myeloid progenitors. Cell 163, 1663–1677 (2015).
Notta, F. et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 351, aab2116 (2016).
Yang, H., Adam, R. C., Ge, Y., Hua, Z. L. & Fuchs, E. Epithelial–mesenchymal micro-niches govern stem cell lineage choices. Cell 169, 483–496.e13 (2017).
Yu, V. W. et al. Epigenetic memory underlies cell-autonomous heterogeneous behavior of hematopoietic stem cells. Cell 168, 944–945 (2017).
Chao, M. P., Seita, J. & Weissman, I. L. Establishment of a normal hematopoietic and leukemia stem cell hierarchy. Cold Spring Harb. Symp. Quant. Biol. 73, 439–449 (2008).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Grun, D. et al. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 525, 251–255 (2015).
Velten, L. et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat. Cell Biol. 19, 271–281 (2017).
Blanpain, C. & Fuchs, E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344, 1242281 (2014).
Muller-Rover, S. et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 117, 3–15 (2001).
Xin, T., Greco, V. & Myung, P. Hardwiring stem cell communication through tissue structure. Cell 164, 1212–1225 (2016).
Greco, V. et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4, 155–169 (2009).
Ito, M., Kizawa, K., Hamada, K. & Cotsarelis, G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation 72, 548–557 (2004).
Rompolas, P., Mesa, K. R. & Greco, V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 502, 513–518 (2013).
Legue, E. & Nicolas, J. F. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development 132, 4143–4154 (2005).
Rompolas, P. et al. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 487, 496–499 (2012).
Seldin, L., Muroyama, A. & Lechler, T. NuMA–microtubule interactions are critical for spindle orientation and the morphogenesis of diverse epidermal structures. eLife 5, e12504 (2016).
Wang, A. B., Zhang, Y. V. & Tumbar, T. Gata6 promotes hair follicle progenitor cell renewal by genome maintenance during proliferation. EMBO J. 36, 61–78 (2017).
Sequeira, I. & Nicolas, J. F. Redefining the structure of the hair follicle by 3D clonal analysis. Development 139, 3741–3751 (2012).
Legue, E., Sequeira, I. & Nicolas, J. F. Hair follicle renewal: authentic morphogenesis that depends on a complex progression of stem cell lineages. Development 137, 569–577 (2010).
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359–363 (2004).
Veniaminova, N. A. et al. Keratin 79 identifies a novel population of migratory epithelial cells that initiates hair canal morphogenesis and regeneration. Development 140, 4870–4880 (2013).
Mesler, A. L., Veniaminova, N. A., Lull, M. V. & Wong, S. Y. Hair follicle terminal differentiation is orchestrated by distinct early and late matrix progenitors. Cell Rep. 19, 809–821 (2017).
Pruitt, S. C., Freeland, A., Rusiniak, M. E., Kunnev, D. & Cady, G. K. Cdkn1b overexpression in adult mice alters the balance between genome and tissue ageing. Nat. Commun. 4, 2626 (2013).
Rompolas, P. et al. Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science 352, 1471–1474 (2016).
Takeda, N. et al. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+ niche cells. Development 140, 1655–1664 (2013).
Choi, Y. S. et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 13, 720–733 (2013).
Kandyba, E. & Kobielak, K. Wnt7b is an important intrinsic regulator of hair follicle stem cell homeostasis and hair follicle cycling. Stem Cells 32, 886–901 (2014).
Gat, U., DasGupta, R., Degenstein, L. & Fuchs, E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 95, 605–614 (1998).
Lo Celso, C., Prowse, D. M. & Watt, F. M. Transient activation of β-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 131, 1787–1799 (2004).
Brown, S. et al. Correction of aberrant growth preserves tissue homeostasis. Nature 548, 334–337 (2017).
Deschene, E. R. et al. β-Catenin activation regulates tissue growth non-cell autonomously in the hair stem cell niche. Science 343, 1353–1356 (2014).
Levy, V., Lindon, C., Harfe, B. D. & Morgan, B. A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell 9, 855–861 (2005).
Harada, N. et al. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 18, 5931–5942 (1999).
Ferrer-Vaquer, A. et al. A sensitive and bright single-cell resolution live imaging reporter of Wnt/β-catenin signaling in the mouse. BMC Dev. Biol. 10, 121 (2010).
Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104, 233–245 (2001).
Blanpain, C. Tracing the cellular origin of cancer. Nat. Cell Biol. 15, 126–134 (2013).
Vaezi, A., Bauer, C., Vasioukhin, V. & Fuchs, E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev. Cell 3, 367–381 (2002).
Rendl, M., Lewis, L. & Fuchs, E. Molecular dissection of mesenchymal–epithelial interactions in the hair follicle. PLoS Biol. 3, e331 (2005).
Vasioukhin, V., Degenstein, L., Wise, B. & Fuchs, E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl Acad. Sci. USA 96, 8551–8556 (1999).
Mesa, K. R. et al. Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature 522, 94–97 (2015).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Harfe, B. D. et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517–528 (2004).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Wang, L. et al. Restricted expression of mutant SOD1 in spinal motor neurons and interneurons induces motor neuron pathology. Neurobiol. Dis. 29, 400–408 (2008).
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Chenn, A. & Walsh, C. A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365–369 (2002).
Acknowledgements
We thank J. Moore and E. Legué for feedback on the manuscript and other Greco lab members for helpful discussion. We thank E. Fuchs for the K14-H2BGFP, pTRE-H2BGFP, K14-actinGFP and K14-Cre mice, and M.Taketo for the β-cateninflox(Ex3) mice. We thank the Yale Transgenic Facility for generating the pTRE-dNβcatGFP mice. This work is supported by the New York Stem Cell Foundation, The Edward Mallinckrodt Jr Foundation, the Glenn Foundation for Medical Research, the HHMI Scholar award, and the National Institute of Arthritis and Musculoskeletal and Skin Disease (NIAMS), NIH, grants no. 2R01AR063663-06A1, no. 1R01AR072668-01 and no. 5R01AR067755-02. V.G. is a New York Stem Cell Foundation Robertson Investigator and HHMI Scholar. T.X. was supported by The James Hudson Brown–Alexander Brown Coxe Postdoctoral Fellowships.
Author information
Authors and Affiliations
Contributions
T.X. and V.G. designed the experiments and wrote the manuscript. T.X. performed the experiments and analysed the data. D.G. assisted with the two-photon imaging and data analysis. P.R. assisted with lineage tracing and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
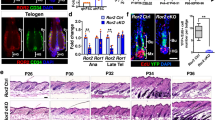
Supplementary Figure 1 Inhibiting proliferation in most stem cell by blocking cell cycle does not affect hair follicle growth rate.
(a), Immunofluorescence staining of early Anagen hair follicles for cell cycle markers Ki67 and BrdU showing the H2BGFP+ cells that also express Cdkn1b in the proliferation inhibition experiments (Lgr5-CreER; R26-flox-STOP-tTA; tetO-Cdkn1b; pTRE-H2BGFP; K14-H2BmCherry) have blocked cell cycle. Images representative of 3 mice. Scale bar, 50 µm. (b), No significant growth rate difference between the Cdkn1b-overexpressing hair follicles carrying non-inhibited stem cells only at a certain position. Plot shows the mean±SD from 3 mice (n=10, n=9 and n=15 hair follicles in each group). ns, not significant, p=0.2385, 0.5210 and 0.4856 when comparing each two groups. Two-sided unpaired t-test was used to calculate p value. Statistical source data are provided in Supplementary Table 1.
Supplementary Figure 2 Photo-inducible labeling and tracing show upward lineage shifting of the matrix progenitors.
Representative images showing lower matrix progenitors labeled by photo-activated H2BmCherry (K14-H2BPAmCherry, red) took over the upper matrix over time through either relocation or expansion. Epithelial membranes were marked by K14-actinGFP (green). Hair follicle epithelium is outlined by white dashed lines. Photo-activated areas are indicated by yellow dashed boxes. H2BmCherry signal was diluted through cell proliferation after photo-activation. Images representative of 7 mice. Scale bar, 20 µm.
Supplementary Figure 3 Lineage tracing of the outer hair follicle layer shows progressively progenitor relocation and lineage change.
Lineage tracing of the outer hair follicle layer, by analyzing non-consecutive sections sampling whole mouse back skin at multiple time points, showing outer cells are progressively relocated towards the upper part of the matrix with corresponding lineage changes. To induce outer layer cell labeling, Lgr5-CreER; R26-flox-STOP-tdTomato; K14-H2BGFP mice were injected a single dose of 2 mg tamoxifen between postnatal day 25 and 26. Back skin was then collected 3 days, 5 days and 7 days after injection, which are referred to as Day 0, 2 and 4. The analyses were typically performed from Anagen V to VI. Mesenchymal dermal papilla was labeled by Lef1-RFP (red). Hair follicle epithelium is outlined by dashed lines in the enlarged images. Scale bars, 100 µm for the large field of view, 20 µm for individual hair follicles. (sample and mouse numbers are indicated in the figure).
Supplementary Figure 4 Outer root sheath cells (ORS) expand and move downwards during hair follicle growth.
(a), Representative images showing lower bulge cells of the resting hair follicle give rise to upper ORS cells which expand and move downwards during hair follicle growth. Images representative of 5 mice. (b), Representative images showing both upper and lower ORS cells expand and move downwards during hair follicle growth. Images representative of 8 mice. Epithelial nuclei were marked by K14-H2BGFP (green). Lgr5-CreER and R26-flox-STOP-tdTomato (red) were used to induce bulge cell or ORS cell labeling. The tracking was performed either from Telogen to Anagen VI (a) or from Anagen IIIc to VI (b). Hair follicle epithelium is outlined by white dashed lines. Scale bar, 20 µm. (c), Frequency of relocation or concurrent multi-lineage differentiation without relocation of the matrix progenitors in total hair follicles analyzed or hair follicles with single progenitor labeled.
Supplementary Figure 5 βCatGOF mutant matrix cells are integrated into the normal differentiation process.
(a), Revisits of a hair follicle where βCatGOF mutation had been induced in both the stem cell compartment and the matrix by Hopx-CreER. While an outgrowth (arrowhead) emerged from the stem cell compartment, the mutant cell in the matrix were gradually relocated into the top matrix area without causing aberrancy. Epithelial nuclei were marked by K14-H2BGFP (green). R26-flox-STOP-tdTomato (red) was used to identify the mutant cells. Asterisk and arrow indicate the original and current progenitor cell position respectively. Images representative of 5 mice. (b), Representative image of hair follicles having GFP-tagged βCatGOF mutation (dNβCatGFP) induced in the stem cells showing ectopic outgrowths (arrowheads and outlined by dashed lines) emerged in the upper part of the hair follicles. Epithelial membrane was marked by K14-ActinmCherry (red). Lgr5-CreER and R26-flox-STOP-tTA were used to induce the dNβCatGFP expression under the Doxycycline-inducible promoter (pTRE-dNβCatGFP) in the stem cells. (c), Revisits of hair follicles where dNβCatGFP had been induced in the matrix. The mutant cells were gradually relocated into the top matrix area and sent progeny upwards to differentiate normally without causing aberrancy. Images representative of 5 mice. Epithelial nuclei were marked by K14-H2BmCherry (red). Shh-CreER and R26-flox-STOP-tTA were used to induce the dNβCatGFP expression. Note that dermal papillae are out of the planes in Day 0 hair follicle images, thus the dashed lines inside the hair follicles do not outline the actual dermal papillae surfaces. Arrows indicate the positions of the individual progenitors labeled. Yellow dashed lines separate different lineages. For a and c, the tracking was performed from Anagen IV to VI and hair follicle epithelium is outlined by white dashed lines. Scale bar, 20 µm.
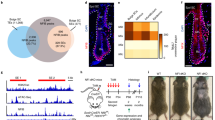
Supplementary Figure 6 Unprocessed original scan of the DNA gel in Fig. 5.
The βCat exon2-4 primers amplify a 363 bp fragment of the wild type β-catenin gene and a 135 bp fragment of the recombined βCatGOF allele.
Supplementary information
Supplementary Information
Supplementary Figures 1–6, Supplementary Table and Supplementary Video legends
Supplementary Table 1
Statistics source data
Supplementary Video 1
3D view and surface rendering of hair follicles from the K14-Cre;mTmG (R26-flox-membrane tdTomato-STOP-membrane GFP) mice showing progressive encapsulation of mesenchymal dermal papilla (red) by epithelium (green) during hair follicle growth
Supplementary Video 2
3D views of the same hair follicle in Telogen and Anagen I from the K14-H2BGFP (green) mice showing there are no cells in the suprabasal space (purple circle) of the Telogen hair germ (white, left), while suprabasal cells (pink highlights one such cell) emerge in the Anagen I hair germ (white, right)
Supplementary Video 3
Time-lapses showing cell death of lower hair germ cells (arrow). Epithelial nuclei were marked by K14-H2BmCherry (left) and K14-H2BGFP (right)
Supplementary Video 4
3D view and surface rendering of a hair follicle during lineage tracing showing suprabasal displacement (blue) of cells deriving from lower stem cell (left) and basal expansion of cells deriving from upper stem cell (right). Epithelial nuclei were marked by K14-H2BGFP (green). Lgr5-CreER and R26-flox-STOP-tdTomato (red) were used to induce stem cell labelling
Supplementary Video 5
3D view and surface rendering of a hair follicle from the Lgr5-CreER; R26-flox-STOP-tTA; tetO-Cdkn1b; pTRE-H2BGFP; K14-H2BmCherry mice showing induction of Cdkn1b and H2BGFP (green, proliferation-impaired cells) expression in all but one (red, proliferation-competent cell) hair germ stem cells
Rights and permissions
About this article
Cite this article
Xin, T., Gonzalez, D., Rompolas, P. et al. Flexible fate determination ensures robust differentiation in the hair follicle. Nat Cell Biol 20, 1361–1369 (2018). https://doi.org/10.1038/s41556-018-0232-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-018-0232-y
This article is cited by
-
Hdac1 and Hdac2 regulate the quiescent state and survival of hair-follicle mesenchymal niche
Nature Communications (2023)
-
Multiphoton intravital microscopy of rodents
Nature Reviews Methods Primers (2022)
-
Skin-resident immune cells actively coordinate their distribution with epidermal cells during homeostasis
Nature Cell Biology (2021)