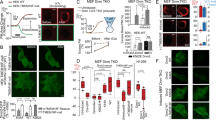
Abstract
The target of rapamycin complex 2 (TORC2) plays a key role in maintaining the homeostasis of plasma membrane (PM) tension. TORC2 activation following increased PM tension involves redistribution of the Slm1 and 2 paralogues from PM invaginations known as eisosomes into membrane compartments containing TORC2. How Slm1/2 relocalization is triggered, and if/how this plays a role in TORC2 inactivation with decreased PM tension, is unknown. Using osmotic shocks and palmitoylcarnitine as orthogonal tools to manipulate PM tension, we demonstrate that decreased PM tension triggers spontaneous, energy-independent reorganization of pre-existing phosphatidylinositol-4,5-bisphosphate into discrete invaginated membrane domains, which cluster and inactivate TORC2. These results demonstrate that increased and decreased membrane tension are sensed through different mechanisms, highlighting a role for membrane lipid phase separation in mechanotransduction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Paluch, E. & Heisenberg, C. P. Biology and physics of cell shape changes in development. Curr. Biol. 19, R790–R799 (2009).
Yu, H., Mouw, J. K. & Weaver, V. M. Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol. 21, 47–56 (2011).
Diz-Munoz, A., Fletcher, D. A. & Weiner, O. D. Use the force: membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 23, 47–53 (2013).
Dai, J. & Sheetz, M. P. Mechanical properties of neuronal growth cone membranes studied by tether formation with laser optical tweezers. Biophys. J. 68, 988–996 (1995).
Lieber, A. D., Yehudai-Resheff, S., Barnhart, E. L., Theriot, J. A. & Keren, K. Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Curr. Biol. 23, 1409–1417 (2013).
Morris, C. E. & Homann, U. Cell surface area regulation and membrane tension. J. Membr. Biol. 179, 79–102 (2001).
Berchtold, D. et al. Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat. Cell Biol. 14, 542–547 (2012).
Walther, T. C. et al. Eisosomes mark static sites of endocytosis. Nature 439, 998–1003 (2006).
Olivera-Couto, A. & Aguilar, P. S. Eisosomes and plasma membrane organization. Mol. Genet. Genom. 287, 607–620 (2012).
Berchtold, D. & Walther, T. C. TORC2 plasma membrane localization is essential for cell viability and restricted to a distinct domain. Mol. Biol. Cell 20, 1565–1575 (2009).
Diz-Munoz, A. et al. Membrane tension acts through PLD2 and mTORC2 to limit actin network assembly during neutrophil migration. PLoS Biol. 14, e1002474 (2016).
Fadri, M., Daquinag, A., Wang, S., Xue, T. & Kunz, J. The pleckstrin homology domain proteins Slm1 and Slm2 are required for actin cytoskeleton organization in yeast and bind phosphatidylinositol-4,5-bisphosphate and TORC2. Mol. Biol. Cell 16, 1883–1900 (2005).
Tabuchi, M., Audhya, A., Parsons, A. B., Boone, C. & Emr, S. D. The phosphatidylinositol 4,5-biphosphate and TORC2 binding proteins Slm1 and Slm2 function in sphingolipid regulation. Mol. Cell Biol. 26, 5861–5875 (2006).
Grossmann, G. et al. Plasma membrane microdomains regulate turnover of transport proteins in yeast. J. Cell Biol. 183, 1075–1088 (2008).
Aronova, S. et al. Regulation of ceramide biosynthesis by TOR complex 2. Cell Metab. 7, 148–158 (2008).
Kamada, Y. et al. Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization. Mol. Cell Biol. 25, 7239–7248 (2005).
Hohmann, S. Control of high osmolarity signalling in the yeast Saccharomyces cerevisiae. FEBS Lett. 583, 4025–4029 (2009).
Brewster, J. L. & Gustin, M. C. Hog1: 20 years of discovery and impact. Sci. Signal 7, re7 (2014).
Levin, D. E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189, 1145–1175 (2011).
Soleimanpour, S. et al. Headgroup engineering in mechanosensitive membrane probes. Chem. Commun. 52, 14450–14453 (2016).
Colom, A. et al. A fluorescent membrane tension probe. Nat. Chem. https://doi.org/10.1038/s41557-018-0127-3 (2018).
Gaubitz, C. et al. Molecular basis of the rapamycin insensitivity of target of rapamycin complex 2. Mol. Cell 58, 977–988 (2015).
Urban, J. et al. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol. Cell 26, 663–674 (2007).
Beese, S. E., Negishi, T. & Levin, D. E. Identification of positive regulators of the yeast fps1 glycerol channel. PLoS Genet. 5, e1000738 (2009).
Novick, P., Ferro, S. & Schekman, R. Order of events in the yeast secretory pathway. Cell 25, 461–469 (1981).
Rispal, D. et al. Target of rapamycin complex 2 regulates actin polarization and endocytosis via multiple pathways. J. Biol. Chem. 290, 14963–14978 (2015).
Desrivieres, S., Cooke, F. T., Parker, P. J. & Hall, M. N. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J. Biol. Chem. 273, 15787–15793 (1998).
Kavran, J. M. et al. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J. Biol. Chem. 273, 30497–30508 (1998).
Stefan, C. J., Audhya, A. & Emr, S. D. The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Mol. Biol. Cell 13, 542–557 (2002).
Dupont, S., Beney, L., Ritt, J. F., Lherminier, J. & Gervais, P. Lateral reorganization of plasma membrane is involved in the yeast resistance to severe dehydration. Biochim. Biophys. Acta 1798, 975–985 (2010).
Singer-Kruger, B., Nemoto, Y., Daniell, L., Ferro-Novick, S. & De Camilli, P. Synaptojanin family members are implicated in endocytic membrane traffic in yeast. J. Cell Sci. 111, 3347–3356 (1998).
Stefan, C. J., Padilla, S. M., Audhya, A. & Emr, S. D. The phosphoinositide phosphatase Sjl2 is recruited to cortical actin patches in the control of vesicle formation and fission during endocytosis. Mol. Cell Biol. 25, 2910–2923 (2005).
Owen, D. M., Williamson, D. J., Magenau, A. & Gaus, K. Sub-resolution lipid domains exist in the plasma membrane and regulate protein diffusion and distribution. Nat. Commun. 3, 1256 (2012).
Sanchez, S. A., Tricerri, M. A. & Gratton, E. Laurdan generalized polarization fluctuations measures membrane packing micro-heterogeneity in vivo. Proc. Natl Acad. Sci. USA 109, 7314–7319 (2012).
Harris, F. M., Best, K. B. & Bell, J. D. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim. Biophys. Acta 1565, 123–128 (2002).
Prouteau, M. et al. TORC1 organized in inhibited domains (TOROIDs) regulate TORC1 activity. Nature 550, 265–269 (2017).
Karuppasamy, M. et al. Cryo-EM structure of Saccharomyces cerevisiae target of rapamycin complex 2. Nat. Commun. 8, 1729 (2017).
Chen, X. et al. Phosphatidylinositol 4,5-bisphosphate clusters the cell adhesion molecule CD44 and assembles a specific CD44–Ezrin heterocomplex, as revealed by small angle neutron scattering. J. Biol. Chem. 290, 6639–6652 (2015).
Honigmann, A. et al. Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nat. Struct. Mol. Biol. 20, 679–686 (2013).
van den Bogaart, G. et al. Membrane protein sequestering by ionic protein–lipid interactions. Nature 479, 552–555 (2011).
Marat, A. L. et al. mTORC1 activity repression by late endosomal phosphatidylinositol 3,4-bisphosphate. Science 356, 968–972 (2017).
Narita, T., Naganuma, T., Sase, Y. & Kihara, A. Long-chain bases of sphingolipids are transported into cells via the acyl-CoA synthetases. Sci. Rep. 6, 25469 (2016).
Loewith, R. & Hall, M. N. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 189, 1177–1201 (2011).
Huber, A. et al. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes Dev. 23, 1929–1943 (2009).
Vincent, J. et al. Small molecule inhibition of cGAS reduces interferon expression in primary macrophages from autoimmune mice. Nat. Commun. 8, 750 (2017).
Wanke, V. et al. Caffeine extends yeast lifespan by targeting TORC1. Mol. Microbiol. 69, 277–285 (2008).
Acknowledgements
The authors thank G. Molinard and M. Prouteau for technical help and T. Kapoor, J. Chiaravalli and J. Fraser Glickman for their collaboration on the high-throughput screen (HTS) campaign. M.R. acknowledges the iGE3 PhD Student Awards. R.L. acknowledges support from the Canton of Geneva, project funding from the Swiss National Science Foundation (SNSF) and the European Research Council Consolidator grant programme. A.R. acknowledges funding from the Swiss National Fund for research grants nos. 31003A_130520, 31003A_149975 and 31003A_173087, and the European Research Council Starting Grant no. 311536 (2011 call). M.R., K.N.-S., A.C., S.S., S.M., A.R. and R.L. are indebted to the National Centre for Competence in Research in Chemical Biology for its support.
Author information
Authors and Affiliations
Contributions
M.R. and K.N.-S. carried out most of the experiments, with the exception of the GUV experiments (N.C.), electron microscopy experiments (B.K. and V.M.) and the screening campaign that led to the identification of PalmC (M.S.). S.S. and S.M. designed and synthetized the FliptR probe, whose properties were characterized by A.C. Finally, M.R., K.N.-S., A.R. and R.L. designed experiments, interpreted results, and wrote the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Independent mechanisms sense increased and decreased PM tension upstream of TORC2.
(a) Hypo-osmotic shock transiently increases TORC2 activity in an amplitude-dependent way. Cells were grown in medium containing 1M sorbitol before being shifted to a lower sorbitol concentration by dilution, and Ypk1 T662 phosphorylation was monitored at the indicated time points. (b) Hyper-osmotic shock transiently decreases TORC2 activity in an amplitude-dependent way. Cells were grown in SC medium before being shifted to a higher sorbitol concentration by dilution, and Ypk1 T662 phosphorylation was monitored at the indicated time points. (c) Quantification of the relative TORC2 activity was calculated for osmotic shocks of increasing amplitude (left, hypo-osmotic shocks; right, hyper-osmotic shocks). Error bars represent the SD of mean values of three independent experiments. (d) Mutations affecting the HOG or CWI pathways do not alter the TORC2 response to osmotic stress. The indicated strains deficient for the HOG and/or the CWI pathway(s) were tested for Ypk1 T662 phosphorylation at the indicated time points after 1M amplitude hypo- (left) or hyper-osmotic shocks (right). All presented blots are representative of results obtained in three independent experiments and all unprocessed scans are shown in Supplementary Figure 7. Source data are included in Supplementary Table 3.
Supplementary Figure 2 Principle of the FLipTR (Fluorescent LIPid Tension Reporter) probe.
(a) Structure of the FLipTR probe. (b) Cartoon illustrating how mechanical forces within the PM affect the twist and polarization of the FLipTR probe.
Supplementary Figure 3 Identification and characterization of Palmitoylcarnitine (PalmC).
(a) Principle of the high-throughput screen leading to the discovery of PalmC. A small molecule library was screened at 10µM on the WT and bypass strains, and compounds having a differential effect on the growth of both strains were selected for further characterization. (b) Comparison between the Normalized Percentage of Inhibition of growth (NPI) of the tested compounds towards the WT and the bypass strain. (c) Structure of PalmC. (d) The TORC2 bypass strain is resistant to PalmC. Strains were grown overnight at 30 °C, diluted to OD600=0.1, regrown into exponential phase and diluted to OD600=0.3. 3μL of 1/5 serial dilutions were then spotted onto plates with or without 20μM PalmC. This experiment was repeated three times with similar results. (e) Mutations affecting the HOG and/or CWI pathways do not alter the TORC2 response to PalmC. The indicated strains were tested for Ypk1 T662 phosphorylation at the indicated time points after PalmC treatment. (f) PalmC does not activate the HOG1 pathway. Hog1 phosphorylation was monitored at the indicated time points after PalmC treatment or hyper-osmotic shock using an anti-phospho-p38 antibody. (g) PalmC and hyper-osmotic shock affect PM tension in an additive manner. Phosphorylation of Ypk1 T662 was monitored in cells treated with a low concentration of PalmC, a low-magnitude hyper-osmotic shock, or a combination of both treatments, at the indicated time points. (h) The effect of PalmC on TORC2 does not require exocytosis. Phosphorylation of Ypk1 T662 was monitored at the indicated time points following PalmC treatment in WT cells or in sec18ts cells deficient for exocytosis27. (i) The effect of PalmC on TORC2 does not require endocytosis. Phosphorylation of Ypk1 T662 was monitored at the indicated time points following PalmC treatment in LatA-treated cells. All blots are representative of at least two independent experiments, and unprocessed scans are shown in Supplementary Figure 7.
Supplementary Figure 4 PES are membrane invaginations, unrelated to endocytic structures.
(a, c) Distributions of the peak intensities of Avo3-GFP (a) or Slm1-mCherry (c) foci (n=100 foci, pooled from three independent experiments) and the thresholds used to define the clusters. (b) A hyper-osmotic shock induces TORC2 (Avo3-GFP) aggregation, but direct inhibition of TORC2 by NVP-BHS345 in TORC1M2282T cells28 does not. Images are maximum projections for 0.5-μm-spaced Z-planes, and representative of three independent experiments; scale bar, 5μm. (d) Illustration of the Rapamycin-mediated anchor-away system. Slm1–FRB dynamically relocalizes to and from eisosomes (left), but irreversible binding of Rapamycin locks it to Sur7–FKBP12 at eisosomes (right). (e) Anchoring of Slm1 to eisosomes impairs TORC2 reactivation following PalmC treatment. WT or TOR1-1 fpr1Δ slm2Δ SLM1–FRB SUR7–2×FKBP12 cells were treated with Rapamycin (5 min), then PalmC, and Ypk1 T662 phosphorylation was monitored. This experiment was repeated twice with similar results, and unprocessed scans of the blots are shown in Supplementary Figure 7. (f) PalmC-treated cells expressing the PtdIns(4,5)P2 biosensor display large PM invaginations enriched in PtdIns(4,5)P2 when visualized by Electron Microscopy after anti-GFP immunogold labelling. For each condition, the middle and right images are a magnification of the indicated region from the left picture and the right image highlights the gold particules in red. Scale bar, 2μm. This experiment was repeated twice with similar results. (g) Filamentous actin is not required for PES formation. Equatorial sections of cells expressing Abp1-mCherry and the GFP-2xPHPLCδ biosensor, treated with 10µM PalmC +/- 2µM LatA. Images are representative from three independent experiments. Scale bar, 5μm. (h) Filamentous actin is not required for PES resolution. Evolution of the percentage of cells displaying PES after PalmC treatment, +/- pre-treatment with LatA. Error bars represent the SD of mean values of three independent experiments (n=50 cells). Source data are included in Supplementary Table 3.
Supplementary Figure 5 The CAAX box rescues TORC2 function in the mss4-103 mutant.
(a) Representative equatorial sections and maximum projections for 0.5µM-spaced Z-planes of mss4-103 cells expressing Avo3-GFP at the permissive (30 °C) and non-permissive (37 °C) temperatures. Scale bar, 5μm. This experiment was repeated three times with similar results. (b) Evolution of TORC2 activity, monitored by Ypk1 T662 phosphorylation, upon shifting WT or mss4-103 cells to the non-permissive temperature. This experiment was repeated twice with similar results. (c) Quantification of TORC2 basal activity, monitored by Ypk1 T662 phosphorylation, in WT and mss4-103 cells at the permissive (30 °C) and non-permissive (37 °C) temperatures. Represented are the mean values +/- SD from three independent experiments and normalized to the WT at 30 °C (*** p<0.001, two-tailed unpaired t-test, see Supplementary Table 3 for the exact p values). Source data are included in Supplementary Table 3. (d) Transient growth at 37 °C does not affect survival of either WT or mss4-103 TORC2CAAX cells. Cells were grown at 37 °C for the indicated times. Serial dilutions of cells were then spotted onto YPD plates and growth was assessed 24h later. This experiment was repeated twice with similar results. All unprocessed scans of blots are shown in Supplementary Figure 7.
Supplementary Figure 6 PES formation does not require de novo PtdIns(4,5)P2 synthesis.
(a) Quantification of the relative PtdIns(4,5)P2 levels at the cortex of the indicated cell upon PalmC treatment. A 2-px-wide line was drawn around the cell perimeter and the GFP-2xPHPLCδ fluorescence intensity was measured. Peaks representing PtdIns(4,5)P2 clusters are marked with asterisks. Presented results are representative of n=20 cells from two independent experiments. (b) Total GFP-2xPHPLCδ fluorescence intensities, expressed as afu/pixel and normalized to the control condition, after 5min PalmC treatment or 1M hyper-osmotic shock. Results are presented as mean +/-SD (n=40 cells pooled from two independent experiments, n.s: not significant change, p>0.05 in two-tailed unpaired t-test, see Supplementary Table 3 for exact p values). Source data are included in Supplementary Table 3. (c) Mss4 activity is necessary for the maintenance of PES. Timelapse of WT or inp51Δinp52Δ cells expressing the GFP-2xPHPLCδ biosensor and treated with 10µM PalmC for 5 min prior to ATP depletion. Images are maximum projections of 0.5μm-spaced Z-planes of the cells, and representative of results observed in three independent experiments. (d) Endocytic BAR protein do not play a major role in PES formation. Representative equatorial sections of cells expressing Rvs167-mCherry and the GFP-2xPHPLCδ biosensor, treated or not for 5min with 10µM PalmC. This experiment was repeated three times with similar results. All scale bars, 5µM.
Supplementary Figure 7
Unprocessed scans of the blots
Supplementary information
Supplementary Information
Supplementary Figures 1–7 and Supplementary Table legends
Supplementary Table 1
Strains used in this study
Supplementary Table 2
Plasmids used in this study
Supplementary Table 3
Statistics source data
Supplementary Table 4
Small molecule screening data
Supplementary Table 5
List of the screen hits
Supplementary Table 6
Primers used in this study
Rights and permissions
About this article
Cite this article
Riggi, M., Niewola-Staszkowska, K., Chiaruttini, N. et al. Decrease in plasma membrane tension triggers PtdIns(4,5)P2 phase separation to inactivate TORC2. Nat Cell Biol 20, 1043–1051 (2018). https://doi.org/10.1038/s41556-018-0150-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-018-0150-z
This article is cited by
-
EGOC inhibits TOROID polymerization by structurally activating TORC1
Nature Structural & Molecular Biology (2023)
-
A molecular mechanosensor for real-time visualization of appressorium membrane tension in Magnaporthe oryzae
Nature Microbiology (2023)
-
Notch ankyrin domain: evolutionary rise of a thermodynamic sensor
Cell Communication and Signaling (2022)
-
Interplay between mechanics and signalling in regulating cell fate
Nature Reviews Molecular Cell Biology (2022)
-
Plant cell polarity as the nexus of tissue mechanics and morphogenesis
Nature Plants (2021)