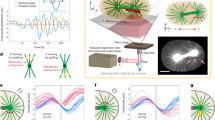
Abstract
Recent advances in cell biology enable precise molecular perturbations. The spatiotemporal organization of cells and organisms, however, also depends on physical processes such as diffusion or cytoplasmic flows, and strategies to perturb physical transport inside cells are not yet available. Here, we demonstrate focused-light-induced cytoplasmic streaming (FLUCS). FLUCS is local, directional, dynamic, probe-free, physiological, and is even applicable through rigid egg shells or cell walls. We explain FLUCS via time-dependent modelling of thermoviscous flows. Using FLUCS, we demonstrate that cytoplasmic flows drive partitioning-defective protein (PAR) polarization in Caenorhabditis elegans zygotes, and that cortical flows are sufficient to transport PAR domains and invert PAR polarity. In addition, we find that asymmetric cell division is a binary decision based on gradually varying PAR polarization states. Furthermore, the use of FLUCS for active microrheology revealed a metabolically induced fluid-to-solid transition of the yeast cytoplasm. Our findings establish how a wide range of transport-dependent models of cellular organization become testable by FLUCS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Turing, A. M. The chemical basis of morphogenesis. Philos. T. R. Soc. Lond. B 237, 37–72 (1952).
Kondo, S. & Miura, T. Reaction–diffusion model as a framework for understanding biological pattern formation. Science 329, 1616–1620 (2010).
Ganguly, S., Williams, L. S., Palacios, I. M. & Goldstein, R. E. Cytoplasmic streaming in Drosophila oocytes varies with kinesin activity and correlates with the microtubule cytoskeleton architecture. Proc. Natl Acad. Sci. USA 109, 15109–15114 (2012).
Goldstein, R. E. & van de Meent, J.-W. A physical perspective on cytoplasmic streaming. Interface Focus. 5, 20150030 (2015).
Howard, J., Grill, S. W. & Bois, J. S. Turing’s next steps: the mechanochemical basis of morphogenesis. Nat. Rev. Mol. Cell Biol 12, 400–406 (2011).
Parry, B. R. et al. The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell 156, 183–194 (2014).
Guo, M. et al. Probing the stochastic, motor-driven properties of the cytoplasm using force spectrum microscopy. Cell 158, 822–832 (2014).
Wolpert, L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 25, 1–47 (1969).
Schwartz, A. L. Cell biology of intracellular protein trafficking. Annu. Rev. Immunol. 8, 195–229 (1990).
Gregor, T., Wieschaus, E. F., McGregor, A. P., Bialek, W. & Tank, D. W. Stability and nuclear dynamics of the bicoid morphogen gradient. Cell 130, 141–152 (2007).
Brangwynne, C. P., Koenderink, G. H., Mackintosh, F. C. & Weitz, D. A. Cytoplasmic diffusion: molecular motors mix it up. J. Cell Biol 183, 583–587 (2008).
Müller, P., Rogers, K. W., Yu, S. R., Brand, M. & Schier, A. F. Morphogen transport. Development 140, 1621–1638 (2013).
Bray, D. & White, J. G. Cortical flow in animal cells. Science 239, 883–888 (1988).
Mayer, M., Depken, M., Bois, J. S., Jülicher, F. & Grill, S. W. Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature 467, 617–621 (2010).
Munro, E., Nance, J. & Priess, J. R. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413–424 (2004).
Goldstein, B. & Macara, I. G. The PAR proteins: fundamental players in animal cell polarization. Dev. Cell 13, 609–622 (2007).
Goehring, N. W. et al. Polarization of PAR proteins by advective triggering of a pattern-forming system. Science 334, 1137–1141 (2011).
Riedel, C. et al. The heat released during catalytic turnover enhances the diffusion of an enzyme. Nature 517, 227–230 (2015).
Munder, M. C. et al. A pH-driven transition of the cytoplasm from a fluid- to a solid-like state promotes entry into dormancy. eLife 5, e09347 (2016).
Joyner, R. P. et al. A glucose-starvation response regulates the diffusion of macromolecules. eLife 5, e09376 (2016).
Weinert, F., Kraus, J., Franosch, T. & Braun, D. Microscale fluid flow induced by thermoviscous expansion along a traveling wave. Phys. Rev. Lett. 100, 164501 (2008).
Weinert, F. M. & Braun, D. Optically driven fluid flow along arbitrary microscale patterns using thermoviscous expansion. J. Appl. Phys. 104, 104701 (2008).
Begasse, M. L., Leaver, M., Vazquez, F., Grill, S. W. & Hyman, A. A. Temperature dependence of cell division timing accounts for a shift in the thermal limits of C. elegans and C. briggsae. Cell. Rep. 10, 647–653 (2015).
Vey, S. & Voigt, A. AMDiS: adaptive multidimensional simulations. Comput. Vis. Sci. 10, 57–67 (2007).
Witkowski, T., Ling, S., Praetorius, S. & Voigt, A. Software concepts and numerical algorithms for a scalable adaptive parallel finite element method. Adv. Comput. Math. 41, 1145–1177 (2015).
Hoege, C. & Hyman, A. A. Principles of PAR polarity in Caenorhabditis elegans embryos. Nat. Rev. Mol. Cell Biol. 14, 315–322 (2013).
Motegi, F. et al. Microtubules induce self-organization of polarized PAR domains in Caenorhabditis elegans zygotes. Nat. Cell Biol. 13, 1361–1367 (2011).
Hird, S. N. & White, J. G. Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J. Cell Biol. 121, 1343–1355 (1993).
Schenk, C., Bringmann, H., Hyman, A. A. & Cowan, C. R. Cortical domain correction repositions the polarity boundary to match the cytokinesis furrow in C. elegans embryos. Development 137, 1743–1753 (2010).
Wallace, E. W. J. et al. Reversible, specific, active aggregates of endogenous proteins assemble upon heat stress. Cell 162, 1286–1298 (2015).
Hu, J. et al. Size- and speed-dependent mechanical behavior in living mammalian cytoplasm. Proc. Natl Acad. Sci. USA 114, 9529–9534 (2017).
Dickinson, D. J., Schwager, F., Pintard, L., Gotta, M. & Goldstein, B. A single-cell biochemistry approach reveals PAR complex dynamics during cell polarization. Dev. Cell 42, 416–434 (2017).
Rodriguez, J. et al. aPKC cycles between functionally distinct PAR protein assemblies to drive cell polarity. Dev. Cell 42, 400–415 (2017).
Wang, S.-C. et al. Cortical forces and CDC-42 control clustering of PAR proteins for Caenorhabditis elegans embryonic polarization Nat. Cell Biol. 19, 988–995 (2017).
Jaeger, J. et al. Dynamic control of positional information in the early Drosophila embryo. Nature 430, 368–371 (2004).
Loose, M., Fischer-Friedrich, E., Ries, J., Kruse, K. & Schwille, P. Spatial regulators for bacterial cell division self-organize into surface waves in vitro. Science 320, 789–792 (2008).
Hecht, I., Rappel, W.-J. & Levine, H. Determining the scale of the bicoid morphogen gradient. Proc. Natl Acad. Sci. USA 106, 1710–1715 (2009).
Rulands, S., Klünder, B. & Frey, E. Stability of localized wave fronts in bistable systems. Phys. Rev. Lett. 110, 038102 (2013).
He, B., Doubrovinski, K., Polyakov, O. & Wieschaus, E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature 508, 392–396 (2014).
Tayar, A. M., Karzbrun, E., Noireaux, V. & Bar-Ziv, R. H. Propagating gene expression fronts in a one-dimensional coupled system of artificial cells. Nat. Phys. 11, 1037–1041 (2015).
Saha, S. et al. Polar positioning of phase-separated liquid compartments in cells regulated by an mRNA competition mechanism. Cell 166, 1572–1584 (2016).
Svitkina, T. M. & Borisy, G. G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 145, 1009–1026 (1999).
Gilleron, J. et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 31, 638–646 (2013).
Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 (2009).
Cremer, T. & Cremer, C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat. Rev. Genet. 2, 292–301 (2001).
Bolzer, A. et al. Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS. Biol. 3, e157 (2005).
Solovei, I. et al. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 137, 356–368 (2009).
Prost, J., Julicher, F. & Joanny, J.-F. Active gel physics. Nat. Phys. 11, 111–117 (2015).
Faubel, R., Westendorf, C., Bodenschatz, E. & Eichele, G. Cilia-based flow network in the brain ventricles. Science 353, 176–178 (2016).
Needleman, D. & Dogic, Z. Active matter at the interface between materials science and cell biology. Nat. Rev. Mater. 2, 17048 (2017).
de Araújo, M. A. C., Silva, R., de Lima, E., Pereira, D. P. & de Oliveira, P. C. Measurement of Gaussian laser beam radius using the knife-edge technique: improvement on data analysis. Appl. Opt. 48, 393–396 (2009).
Duhr, S. & Braun, D. Why molecules move along a temperature gradient. Proc. Natl Acad. Sci. USA 103, 19678–19682 (2006).
Hannak, E. & Heald, R. Investigating mitotic spindle assembly and function in vitro using Xenopus laevis egg extracts. Nat. Protoc. 1, 2305–2314 (2006).
Mittasch, M. et al. How to apply FLUCS in single cells and living embryos. Nat. Protoc. Exch. https://doi.org/10.1038/protex.2017.157 (2017).
Schonegg, S., Constantinescu, A. T. & Hoege, C. & Hyman, A. A. The Rho GTPase-activating proteins RGA-3 and RGA-4 are required to set the initial size of PAR domains in Caenorhabditis elegans one-cell embryos. Proc. Natl Acad. Sci. USA 104, 14976–14981 (2007).
Reymann, A.-C., Staniscia, F., Erzberger, A., Salbreux, G. & Grill, S. W. Cortical flow aligns actin filaments to form a furrow. eLife 5, e17807 (2016).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Serrano, R. Energy requirements for maltose transport in yeast. Eur. J. Biochem. 80, 97–102 (1977).
Peterman, E. J. G., Gittes, F. & Schmidt, C. F. Laser-induced heating in optical traps. Biophys. J. 84, 1308–1316 (2003).
Thielicke, W. & Stamhuis, E. J. PIVlab—towards user-friendly, affordable and accurate digital particle image velocimetry in MATLAB. J. Open Res. Softw. 2, 1202–1210 (2014).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Sbalzarini, I. F. & Koumoutsakos, P. Feature point tracking and trajectory analysis for video imaging in cell biology. J. Struct. Biol. 151, 182–195 (2005).
Petrásek, Z. et al. Characterization of protein dynamics in asymmetric cell division by scanning fluorescence correlation spectroscopy. Biophys. J. 95, 5476–5486 (2008).
Tarantino, N. TNF and IL-1 exhibit distinct ubiquitin requirements for inducing NEMO–IKK supramolecular structures. J. Cell Biol. 204, 231–245 (2014).
Acknowledgements
The authors acknowledge support from the Max Planck Society, a DFG-financed DIPP fellowship for M.Mi., infrastructural support by the Hyman lab, and technical support from MPI-CBG light-microscopy and scientific computing facilities. The authors thank F. Decker and J. Brugués for egg extracts, H. Petzold for cell culture support, D.J. Dickinson and B. Goldstein for sharing transgenic C. elegans strains, and M. Zerial, D. Braun, P. Tomancak, F. Jülicher, A. Hyman, C. Hoege, J. Saenz, K. Subramanian, N. Maghelli and E. Knust for discussions and comments.
Author information
Authors and Affiliations
Contributions
M.Mi., A.W.F. and M.Kr. designed the experimental set-up. M.Mi. conducted experiments. M.Mi. and M.Kr. analysed data. M.Mi. wrote the initial draft. P.G. assisted with C. elegans experiments and provided worm lines. M.N. and A.V. performed finite-element simulations. C.I. and M.Mu. helped with yeast measurements. M.Ka. assisted with gel chemistry. M.Mi., P.G., A.W.F., S.W.G. and M.Kr. conceived and interpreted C. elegans experiments, M.Mi., S.A. and M.Kr. conceived and interpreted yeast experiments. All authors contributed to a critical discussion of the data and participated in writing the manuscript, which M.Mi. and M.Kr. finalized. M.Kr. coordinated the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare that parts of the published work led to the application for a European patent.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–6 and Supplementary Video legends.
Videos
Supplementary Video 1
Light-induced thermoviscous flows in water and honey.
Supplementary Video 2
Directional transport perturbation in Xenopus laevis egg extract.
Supplementary Video 3
Dynamic and localized induction of flows inside C. elegans embryos.
Supplementary Video 4
Quantification of sub-millisecond temperature dynamics.
Supplementary Video 5
Viability of C. elegans embryos in response to dynamic laser-induced heating.
Supplementary Video 6
Flow-driven PAR-2 loading enhancement on the membrane in polarized C. elegans embryos.
Supplementary Video 7
Flow-driven dynamic translocation of the PAR-2 domain in a polarized C. elegans embryo.
Supplementary Video 8
Induced cytoplasmic flows drive flows of the actomyosin cortex.
Supplementary Video 9
PAR polarization of the C. elegans embryo is a bi-stable process.
Supplementary Video 10
Micro-rheological flow stimulus.
Rights and permissions
About this article
Cite this article
Mittasch, M., Gross, P., Nestler, M. et al. Non-invasive perturbations of intracellular flow reveal physical principles of cell organization. Nat Cell Biol 20, 344–351 (2018). https://doi.org/10.1038/s41556-017-0032-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-017-0032-9
This article is cited by
-
Opto-fluidically multiplexed assembly and micro-robotics
Light: Science & Applications (2024)
-
ISO-FLUCS: symmetrization of optofluidic manipulations in quasi-isothermal micro-environments
eLight (2023)
-
Advancing optothermal manipulation: decoupling temperature and flow fields in quasi-isothermal microscale streaming
Light: Science & Applications (2023)
-
Modulating biomolecular condensates: a novel approach to drug discovery
Nature Reviews Drug Discovery (2022)
-
Charge-density reduction promotes ribozyme activity in RNA–peptide coacervates via RNA fluidization and magnesium partitioning
Nature Chemistry (2022)