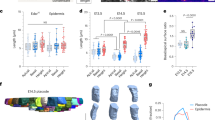
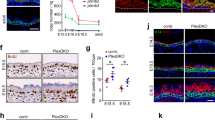
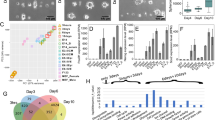
Abstract
To establish and maintain organ structure and function, tissues need to balance stem cell proliferation and differentiation rates and coordinate cell fate with position. By quantifying and modelling tissue stress and deformation in the mammalian epidermis, we find that this balance is coordinated through local mechanical forces generated by cell division and delamination. Proliferation within the basal stem/progenitor layer, which displays features of a jammed, solid-like state, leads to crowding, thereby locally distorting cell shape and stress distribution. The resulting decrease in cortical tension and increased cell–cell adhesion trigger differentiation and subsequent delamination, reinstating basal cell layer density. After delamination, cells establish a high-tension state as they increase myosin II activity and convert to E-cadherin-dominated adhesion, thereby reinforcing the boundary between basal and suprabasal layers. Our results uncover how biomechanical signalling integrates single-cell behaviours to couple proliferation, cell fate and positioning to generate a multilayered tissue.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
06 July 2018
In the version of this Article originally published, Supplementary Video 1 was incorrectly linked to Supplementary Video 6, Supplementary Video 2 was incorrectly linked to Supplementary Video 1, Supplementary Video 3 was incorrectly linked to Supplementary Video 2, Supplementary Video 4 was incorrectly linked to Supplementary Video 3, Supplementary Video 5 was incorrectly linked to Supplementary Video 4, and Supplementary Video 6 was incorrectly linked to Supplementary Video 5. The files have now been replaced to rectify this.
References
Heisenberg, C. P. & Bellaiche, Y. Forces in tissue morphogenesis and patterning. Cell 153, 948–962 (2013).
Keller, R. Developmental biology. Physical biology returns to morphogenesis. Science 338, 201–203 (2012).
Lecuit, T. & Lenne, P. F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell. Biol. 8, 633–644 (2007).
Blanpain, C. & Fuchs, E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell. Biol. 10, 207–217 (2009).
Roshan, A. & Jones, P. H. Act your age: tuning cell behavior to tissue requirements in interfollicular epidermis. Semin. Cell. Dev. Biol. 23, 884–889 (2012).
Clayton, E. et al. A single type of progenitor cell maintains normal epidermis. Nature 446, 185–189 (2007).
Mascre, G. et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489, 257–262 (2012).
Rompolas, P. et al. Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science 502, 513–518 (2016).
Bi, D. P., Yang, X. B., Marchetti, M. C. & Manning, M. L. Motility-driven glass and jamming transitions in biological tissues. Phys. Rev. X 6, 021011 (2016).
Garcia, S. et al. Physics of active jamming during collective cellular motion in a monolayer. Proc. Natl Acad. Sci. USA 112, 15314–15319 (2015).
Merkel, M. & Manning, M. L. Using cell deformation and motion to predict forces and collective behavior in morphogenesis. Semin. Cell. Dev. Biol. 67, 161–169 (2016).
Riedl, J. et al. Lifeact mice for studying F-actin dynamics. Nat. Methods 7, 168–169 (2010).
Luxenburg, C. et al. Wdr1-mediated cell shape dynamics and cortical tension are essential for epidermal planar cell polarity. Nat. Cell. Biol. 17, 592–604 (2015).
Botchkarev, V. A. & Flores, E. R. p53/p63/p73 in the epidermis in health and disease. Cold Spring Harb. Perspect. Med. 4, a015248 (2014).
Farhadifar, R., Roper, J. C., Aigouy, B., Eaton, S. & Julicher, F. The influence of cell mechanics, cell–cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095–2104 (2007).
Marinari, E. et al. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 484, 542–545 (2012).
Sugimura, K. & Ishihara, S. The mechanical anisotropy in a tissue promotes ordering in hexagonal cell packing. Development 140, 4091–4101 (2013).
Mandal, K., Wang, I., Vitiello, E., Orellana, L. A. & Balland, M. Cell dipole behaviour revealed by ECM sub-cellular geometry. Nat. Commun. 5, 5749 (2014).
Thery, M. et al. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc. Natl Acad. Sci. USA 103, 19771–19776 (2006).
De Mets, R., Hennig, K., Bureau, L. & Balland, M. Fast and robust fabrication of reusable molds for hydrogel micro-patterning. Biomater. Sci. 4, 1630–1637 (2016).
Tseng, Q. et al. Spatial organization of the extracellular matrix regulates cell–cell junction positioning. Proc. Natl Acad. Sci. USA 109, 1506–1511 (2012).
Sabass, B., Gardel, M. L., Waterman, C. M. & Schwarz, U. S. High resolution traction force microscopy based on experimental and computational advances. Biophys. J. 94, 207–220 (2008).
Tseng, Q. et al. A new micropatterning method of soft substrates reveals that different tumorigenic signals can promote or reduce cell contraction levels. Lab. Chip. 11, 2231–2240 (2011).
Muller, D. J. & Dufrene, Y. F. Atomic force microscopy: a nanoscopic window on the cell surface. Trends Cell. Biol. 21, 461–469 (2011).
Lomakina, E. B., Spillmann, C. M., King, M. R. & Waugh, R. E. Rheological analysis and measurement of neutrophil indentation. Biophys. J. 87, 4246–4258 (2004).
Salbreux, G., Charras, G. & Paluch, E. Actin cortex mechanics and cellular morphogenesis. Trends Cell. Biol. 22, 536–545 (2012).
Le, H. Q. et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 18, 864–875 (2016).
Paterson, H. F. et al. Microinjection of recombinant p21ρ induces rapid changes in cell morphology. J. Cell Biol. 111, 1001–1007 (1990).
Park, J. A. et al. Unjamming and cell shape in the asthmatic airway epithelium. Nat. Mater. 14, 1040–1048 (2015).
Ranft, J. et al. Fluidization of tissues by cell division and apoptosis. Proc. Natl Acad. Sci. USA 107, 20863–20868 (2010).
Su,T. & Lan, G. Overcrowding drives the unjamming transition of gap-free monolayers. Preprint at https://arxiv.org/ftp/arxiv/papers/1610/1610.04254.pdf (2016).
Guillot, C. & Lecuit, T. Adhesion disengagement uncouples intrinsic and extrinsic forces to drive cytokinesis in epithelial tissues. Dev. Cell. 24, 227–241 (2013).
Herszterg, S., Leibfried, A., Bosveld, F., Martin, C. & Bellaiche, Y. Interplay between the dividing cell and its neighbors regulates adherens junction formation during cytokinesis in epithelial tissue. Dev. Cell. 24, 256–270 (2013).
Lukinavicius, G. et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat. Methods 11, 731–733 (2014).
Youssef, J., Nurse, A. K., Freund, L. B. & Morgan, J. R. Quantification of the forces driving self-assembly of three-dimensional microtissues. Proc. Natl Acad. Sci. USA 108, 6993–6998 (2011).
Dzementsei, A., Schneider, D., Janshoff, A. & Pieler, T. Migratory and adhesive properties of Xenopus laevis primordial germ cells in vitro. Biol. Open 2, 1279–1287 (2013).
Watt, F. M. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 21, 3919–3926 (2002).
Lien, W. H., Klezovitch, O. & Vasioukhin, V. Cadherin-catenin proteins in vertebrate development. Curr. Opin. Cell. Biol. 18, 499–506 (2006).
Tunggal, J. A. et al. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 24, 1146–1156 (2005).
Wheelock, M. J. & Jensen, P. J. Regulation of keratinocyte intercellular junction organization and epidermal morphogenesis by E-cadherin. J. Cell. Biol. 117, 415–425 (1992).
Tinkle, C. L., Lechler, T., Pasolli, H. A. & Fuchs, E. Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc. Natl Acad. Sci. USA 101, 552–557 (2004).
Charest, J. L., Jennings, J. M., King, W. P., Kowalczyk, A. P. & Garcia, A. J. Cadherin-mediated cell–cell contact regulates keratinocyte differentiation. J. Invest. Dermatol. 129, 564–572 (2009).
Hines, M. D., Jin, H. C., Wheelock, M. J. & Jensen, P. J. Inhibition of cadherin function differentially affects markers of terminal differentiation in cultured human keratinocytes. J. Cell. Sci. 112, 4569–4579 (1999).
Galle, J., Hoffmann, M. & Aust, G. From single cells to tissue architecture-a bottom-up approach to modelling the spatio-temporal organisation of complex multi-cellular systems. J. Math. Biol. 58, 261–283 (2009).
Galle, J., Loeffler, M. & Drasdo, D. Modeling the effect of deregulated proliferation and apoptosis on the growth dynamics of epithelial cell populations in vitro. Biophys. J. 88, 62–75 (2005).
Maitre, J. L. et al. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science 338, 253–256 (2012).
Potten, C. S. et al. Proliferation in murine epidermis after minor mechanical stimulation. Part 1. Sustained increase in keratinocyte production and migration. Cell. Prolif. 33, 231–246 (2000).
Connelly, J. T. et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat. Cell. Biol. 12, 711–718 (2010).
Watt, F. M., Jordan, P. W. & O’Neill, C. H. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc. Natl Acad. Sci. USA 85, 5576–5580 (1988).
Eisenhoffer, G. T. & Rosenblatt, J. Bringing balance by force: live cell extrusion controls epithelial cell numbers. Trends Cell. Biol. 23, 185–192 (2013).
Saw, T. B. et al. Topological defects in epithelia govern cell death and extrusion. Nature 544, 212–216 (2017).
Amack, J. D. & Manning, M. L. Knowing the boundaries: extending the differential adhesion hypothesis in embryonic cell sorting. Science 338, 212–215 (2012).
Krieg, M. et al. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 10, 429–436 (2008).
Manning, M. L., Foty, R. A., Steinberg, M. S. & Schoetz, E. M. Coaction of intercellular adhesion and cortical tension specifies tissue surface tension. Proc. Natl Acad. Sci. USA 107, 12517–12522 (2010).
Winklbauer, R. Cell adhesion strength from cortical tension—an integration of concepts. J. Cell. Sci. 128, 3687–3693 (2015).
Gautrot, J. E. et al. Mimicking normal tissue architecture and perturbation in cancer with engineered micro-epidermis. Biomaterials 33, 5221–5229 (2012).
Steinberg, M. S. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science 141, 401–408 (1963).
Bazellieres, E. et al. Control of cell–cell forces and collective cell dynamics by the intercellular adhesome. Nat. Cell Biol. 17, 409–420 (2015).
Niessen, C. M., Leckband, D. & Yap, A. S. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol. Rev. 91, 691–731 (2011).
Rübsam, M. et al. E-cadherin integrates mechanotransduction and EGFR signaling to control junctional tissue polarization and barrier formation. Nat. Commun. 8, 1250 (2017).
Seltmann, K., Fritsch, A. W., Kas, J. A. & Magin, T. M. Keratins significantly contribute to cell stiffness and impact invasive behavior. Proc. Natl Acad. Sci. USA 110, 18507–18512 (2013).
Johnson, J. L., Najor, N. A. & Green, K. J. Desmosomes: regulators of cellular signaling and adhesion in epidermal health and disease. Cold Spring Harb. Perspect. Med. 4, a015297 (2014).
Sumigray, K. D. & Lechler, T. Cell adhesion in epidermal development and barrier formation. Curr. Top. Dev. Biol. 112, 383–414 (2015).
Aigouy, B. et al. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142, 773–786 (2010).
Azioune, A., Carpi, N., Tseng, Q., Thery, M. & Piel, M. Protein micropatterns: a direct printing protocol using deep UVs. Methods Cell. Biol. 97, 133–146 (2010).
Maruthamuthu, V., Sabass, B., Schwarz, U. S. & Gardel, M. L. Cell-ECM traction force modulates endogenous tension at cell–cell contacts. Proc. Natl Acad. Sci. USA 108, 4708–4713 (2011).
Schneider, D. et al. Tension monitoring during epithelial-to-mesenchymal transition links the switch of phenotype to expression of moesin and cadherins in NMuMG cells. PLoS. ONE 8, e80068 (2013).
Pietuch, A., Bruckner, B. R., Fine, T., Mey, I. & Janshoff, A. Elastic properties of cells in the context of confluent cell monolayers: impact of tension and surface area regulation. Soft Matter 9, 11490–11502 (2013).
Arnette, C., Koetsier, J. L., Hoover, P., Getsios, S. & Green, K. J. In vitro model of the epidermis: connecting protein function to 3D structure. Methods Enzymol. 569, 287–308 (2016).
Acknowledgements
We thank V. Braga, R. Fässler and P. H. Jones for critical reading of manuscript, R. Fässler for support with micropatterning, E. Bodenschatz for the AFM, R. Wedlich-Söldner for the LifeAct mice, and the FACS & Imaging Core Facility of MPI for Biology of Ageing for support with imaging. The computations were performed on a Bull Cluster at the Center for Information Services and High Performance Computing (ZIH) at TU Dresden. This work was supported by the Max Planck Society, the Max Planck Förderstiftung, the Behrens-Weise Foundation (to S.A.W.) and Deutsche Forschungsgemeinschaft through SFB 829 (to C.M.N. and S.A.W.), through SFB 937 (to N.K. and M.T.), and through SPP1782 (to C.M.N.), by the Whitaker postdoctoral fellowship (to Y.A.M.), and by the BMBF grant INDRA (031A312 to J.G.).
Author information
Authors and Affiliations
Contributions
S.A.W. conceived and supervised the study. Y.A.M., D.S., H.Q.L., M.R. and N.B. performed experiments and analysed data. T.T. and J.G. designed, performed and analysed the computer simulations. J.P. assisted with initial design and production of micropatterns. N.K. and M.T. performed cell–cell adhesion force measurements. I.W. and M.B. provided algorithms and analyses of traction force and monolayer flow experiments, C.M.N. designed experiments and analysed data. S.A.W. designed and performed experiments, analysed data and wrote the paper. All authors commented on and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures and Legends, Supplementary Table Legends, Supplementary Video Legends, References.
Supplementary Table 1
Parameter set of the model epidermis.
Supplementary Table 2
Statistics source data.
Videos
Supplementary Video 1
Time-lapse confocal video of LifeAct E15.5 embryo with vectors and smoothed velocity map. Images of back skin of embryos were acquired every 10 mins and subjected to PIV analyses. Motility vectors (left panel) and smoothed velocity maps (right panel) are shown.
Supplementary Video 2
Time-lapse confocal video of LifeAct E15.5 embryo. Images were acquired every 10 mins. Asterisks demarcate a dividing cell and arrowhead a delaminating cell.
Supplementary Video 3
Time-lapse DIC video and smoothed velocity map of EPC monolayers after Ca2+ treatment. Images of EPC monolayers treated with 1.8 mM Ca2+ were acquired every 20 mins and subjected to PIV analyses. Motility vectors (left panel) and smoothed velocity maps (right panel) are shown. Asterisks demarcate examples of 2 dividing cells and arrowhead an example of a delaminating cell.
Supplementary Video 4
Time-lapse DIC video of Ca2+ treated EPCs on a circular micropattern. Images were acquired every 10 mins and the video is shown 1 frame/sec.
Supplementary Video 5
Time-lapse video of a 3D model epidermis simulation. A side view is shown.
Supplementary Video 6
Time-lapse video of a 3D model epidermis simulation. A top view is shown.
Rights and permissions
About this article
Cite this article
Miroshnikova, Y.A., Le, H.Q., Schneider, D. et al. Adhesion forces and cortical tension couple cell proliferation and differentiation to drive epidermal stratification. Nat Cell Biol 20, 69–80 (2018). https://doi.org/10.1038/s41556-017-0005-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-017-0005-z
This article is cited by
-
Mechanical state transitions in the regulation of tissue form and function
Nature Reviews Molecular Cell Biology (2024)
-
Mechanical forces across compartments coordinate cell shape and fate transitions to generate tissue architecture
Nature Cell Biology (2024)
-
TRIM40 is a pathogenic driver of inflammatory bowel disease subverting intestinal barrier integrity
Nature Communications (2023)
-
Directionality of developing skeletal muscles is set by mechanical forces
Nature Communications (2023)
-
Contact Guidance Drives Upward Cellular Migration at the Mesoscopic Scale
Cellular and Molecular Bioengineering (2023)