Abstract
Monitoring X-ray radiation in the gastrointestinal tract can enhance the precision of radiotherapy in patients with gastrointestinal cancer. Here we report the design and performance, in the gastrointestinal tract of rabbits, of a swallowable X-ray dosimeter for the simultaneous real-time monitoring of absolute absorbed radiation dose and of changes in pH and temperature. The dosimeter consists of a biocompatible optoelectronic capsule containing an optical fibre, lanthanide-doped persistent nanoscintillators, a pH-sensitive polyaniline film and a miniaturized system for the wireless readout of luminescence. The persistent luminescence of the nanoscintillators after irradiation can be used to continuously monitor pH without the need for external excitation. By using a neural-network-based regression model, we estimated the radiation dose from radioluminescence and afterglow intensity and temperature, and show that the dosimeter was approximately five times more accurate than standard methods for dose determination. Swallowable dosimeters may help to improve radiotherapy and to understand how radiotherapy affects tumour pH and temperature.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
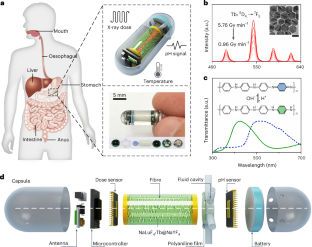




Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. All data generated in this study, including source data and the data used to make the figures, are available for research purposes from the corresponding author on reasonable request.
Code availability
The code is available from https://github.com/yly1994/Swallowable-X-ray-Dosimeter-.git.
References
Steiger, C. et al. Ingestible electronics for diagnostics and therapy. Nat. Rev. Mater. 4, 83–98 (2018).
Huynh, E. et al. Artificial intelligence in radiation oncology. Nat. Rev. Clin. Oncol. 17, 771–781 (2020).
Mijnheer, B., Beddar, S., Izewska, J. & Reft, C. In vivo dosimetry in external beam radiotherapy. Med. Phys. 40, 070903 (2013).
Patel, R. B. et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci. Transl. Med. 13, eabb3631 (2021).
Soliman, Y. S., El Gohary, M. I., Abdel Gawad, M. H., Amin, E. A. & Desouky, O. S. Fricke gel dosimeter as a tool in quality assurance of the radiotherapy treatment plans. Appl. Radiat. Isot. 120, 126–132 (2017).
Chaikh, A., Beuve, M. & Balosso, J. Nanotechnology in radiation oncology: the need for implantable nano dosimeters for in-vivo real time measurements. Int. J. Cancer Ther. Oncol. 3, 3217 (2015).
O’Keeffe, S. et al. A review of recent advances in optical fibre sensors for in vivo dosimetry during radiotherapy. Br. J. Radiol. 88, 20140702 (2015).
Dai, Y. H. et al. Radiosensitivity index emerges as a potential biomarker for combined radiotherapy and immunotherapy. NPJ Genom. Med. 6, 40 (2021).
Yang, Y. et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 38, 217–224 (2020).
Wang, C. et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2, 687–695 (2018).
Tang, B. et al. A near-infrared neutral pH fluorescent probe for monitoring minor pH changes: imaging in living HepG2 and HL-7702 cells. J. Am. Chem. Soc. 131, 3016–3023 (2009).
Zhuang, Q. et al. Embedded structure fiber-optic radiation dosimeter for radiotherapy applications. Opt. Express 24, 5172–5185 (2016).
Piermattei, A. et al. A National project for in vivo dosimetry procedures in radiotherapy: first results. Nucl. Instrum. Methods Phys. Res. B 274, 42–50 (2012).
Yu, G. et al. A discrete organoplatinum(II) metallacage as a multimodality theranostic platform for cancer photochemotherapy. Nat. Commun. 9, 4335 (2018).
Klein, D. et al. In-phantom dose verification of prostate IMRT and VMAT deliveries using plastic scintillation detectors. Radiat. Meas. 47, 921–929 (2012).
Tanderup, K., Beddar, S., Andersen, C. E., Kertzscher, G. & Cygler, J. E. In vivo dosimetry in brachytherapy. Med. Phys. 40, 070902 (2013).
Scarantino, C. W. et al. An implantable radiation dosimeter for use in external beam radiation therapy. Med. Phys. 31, 2658–2671 (2004).
Beyer, G. P. et al. An implantable MOSFET dosimeter for the measurement of radiation dose in tissue during cancer therapy. IEEE Sens. J. 8, 38–51 (2008).
Abramson, A. et al. Oral delivery of systemic monoclonal antibodies, peptides and small molecules using gastric auto-injectors. Nat. Biotechnol. 40, 103–109 (2022).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
Al-Rawhani, M. A., Beeley, J. & Cumming, D. R. Wireless fluorescence capsule for endoscopy using single photon-based detection. Sci. Rep. 5, 18591 (2015).
Nadeau, P. et al. Prolonged energy harvesting for ingestible devices. Nat. Biomed. Eng. 1, 0022 (2017).
Kalantar-Zadeh, K. et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat. Electron. 1, 79–87 (2018).
Ou, J. Z. et al. Potential of in vivo real-time gastric gas profiling: a pilot evaluation of heat-stress and modulating dietary cinnamon effect in an animal model. Sci. Rep. 6, 33387 (2016).
Iacovacci, V. et al. A fully implantable device for intraperitoneal drug delivery refilled by ingestible capsules. Sci. Robot. 6, eabh3328 (2021).
Abramson, A. et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 363, 611–615 (2019).
Mimee, M. et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science 360, 915–918 (2018).
Kimchy, Y. et al. Radiographic capsule-based system for non-cathartic colorectal cancer screening. Abdom. Radiol. 42, 1291–1297 (2017).
Khutoryanskiy, V. V. Supramolecular materials: longer and safer gastric residence. Nat. Mater. 14, 963–964 (2015).
Gora, M. J. et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat. Med. 19, 238–240 (2013).
Lee, H. et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 11, 566–572 (2016).
Sonmezoglu, S., Fineman, J. R., Maltepe, E. & Maharbiz, M. M. Monitoring deep-tissue oxygenation with a millimeter-scale ultrasonic implant. Nat. Biotechnol. 39, 855–864 (2021).
Waltz, E. Drugs go wireless. Nat. Biotechnol. 34, 15–18 (2016).
Qureshi, W. A. Current and future applications of the capsule camera. Nat. Rev. Drug. Discov. 4, 447–450 (2004).
Smith, S., Oberholzer, A., Korvink, J. G., Mager, D. & Land, K. Wireless colorimetric readout to enable resource-limited point-of-care. Lab Chip 19, 3344–3353 (2019).
Kim, T., McCall, J. G. & Jung, Y. H. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Ou, X. et al. High-resolution X-ray luminescence extension imaging. Nature 590, 410–415 (2021).
Ma, G. et al. pH-responsive nanoprobe for in vivo photoacoustic imaging of gastric acid. Anal. Chem. 91, 13570–13575 (2019).
Nakata, S. et al. A wearable pH sensor with high sensitivity based on a flexible charge-coupled device. Nat. Electron. 1, 596–603 (2018).
Anemone, A., Consolino, L., Arena, F., Capozza, M. & Longo, D. L. Imaging tumor acidosis: a survey of the available techniques for mapping in vivo tumor pH. Cancer Metastasis Rev. 38, 25–49 (2019).
Berger, M. J. Xcom: Photon Cross Sections Database (NIST, 2013); https://www.nist.gov/pml/xcom-photon-cross-sections-database
Abu-Thabit, N., Umar, Y., Ratemi, E., Ahmad, A. & Ahmad Abuilaiwi, F. A flexible optical pH sensor based on polysulfone membranes coated with pH-responsive polyaniline nanofibers. Sensors 16, 986 (2016).
Li, J. et al. Functional photoacoustic imaging of gastric acid secretion using pH-responsive polyaniline nanoprobes. Small 12, 4690–4696 (2016).
Hartings, M. R., Castro, N. J., Gill, K. & Ahmed, Z. A photonic pH sensor based on photothermal spectroscopy. Sens. Actuators B 301, 127076 (2019).
Acknowledgements
This work was supported by the NUS NANONASH Program (NUHSRO/2020/002/413 NanoNash/LOA; R143000B43114), National Research Foundation, Prime Minister’s Office, Singapore under its Competitive Research Program (CRP Award No. NRF-NRFI05-2019-0003), the Natural Science Foundation of China (92159304, 82171958, 81901812), Industrial Technology Innovation Project of Suzhou (SYG201919), Science and Technology Key Project of Shenzhen (JCYJ20190812163614809, JCYJ20200109114612308), Singapore National Medical Research Council (NMRC/OFYIRG/0081/2018) and NUS ODPRT Cross-Faculty Research Fund (CFGFY20P14). We thank Z. Liu and H. Tan for technical assistance.
Author information
Authors and Affiliations
Contributions
L.Y., B.H. and X.L. conceived and designed the project. X.L., B.Z., Z.S. and R.Z. supervised the project and guided the collaboration. L.Y. characterized the materials and conducted numerical simulations. C.L and B.X. performed electrical device fabrication. Z.S., B.H., D.H., Z.L. and D.G. performed in vivo experiments. B.H. and L.Y. performed luminescence measurements and experimental validation. J.-W.W. and C.N.L. contributed to the device design. B.H. and L.Y. wrote the manuscript. B.H., L.Y. and X.L. edited the manuscript. All authors participated in the discussion and analyses reported in the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Louis Archambault, Christopher Bettinger, David Gladstone and Kyoungtae Lee for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Synthesis and characterization of Tb3+-doped X-ray scintillation nanocrystals.
a, TEM images of hexagonal-phase NaLuF4:Tb nanocrystals synthesized in various sizes (top) and corresponding radioluminescence images (bottom). b, X-ray energy-dependent absorption spectra of SrAl2O4, ZnS, and NaLuF4. Attenuation coefficients were obtained from ref. 39. The inset shows a schematic of X-ray-induced photoionization. c, Powder X-ray diffraction patterns of NaLuF4:Tb nanocrystals. All peaks are consistent with the hexagonal-phase NaLuF4 structure (Joint Committee on Powder Diffraction Standards (PDF) file number 27-0726, https://github.com/yly1994/Swallowable-X-ray-Dosimeter-.git). d, EDX spectra of NaLuF4:Tb nanocrystals.
Extended Data Fig. 2 Characterization of the NaLuF4:Tb@NaYF4-embedded optical fibre.
a, Radioluminescence and afterglow photographs of NaLuF4:Tb@NaYF4 (15 mol%) nanocrystals embedded in an optical fibre. X-ray operation was set to 50 kV, with tube currents of 80 μA (16.68 mGy/min) and 20 μA (4.17 mGy/min). b, Radioluminescence spectra of the fibre embedded with NaLuF4:Tb@NaYF4(15 mol%) nanocrystals of different doping concentrations. c, Decay curves of NaLuF4:Tb@NaYF4 (15 mol%) nanocrystals after exposure to various X-ray doses at room temperature. d, Radioluminescence intensity versus dose rate at different wavelengths. e, Comparison of radioluminescence of various X-ray scintillators after termination of X-ray irradiation (50 kV and 5 kV). f, Time-dependent X-ray luminescence intensity at various temperatures. g, Radioluminescence intensity of NaLuF4:Tb@NaYF4 nanocrystals when irradiated with X-rays at 2, 3 and 5 Hz.
Extended Data Fig. 3 Comparison of optical fibre designs with various geometric dimensions.
a, Light intensity distribution of the end of an optical fibre made of thermoplastic silicone with a radius of 2 mm and a length of 8 mm, in which a cylindrical ring made of a luminescent material with a thickness of 300 μm is embedded. b and c, Simulations of an optical fibre containing a cylindrical luminescent core with a diameter of 1 mm and irradiated with X-rays from the left (b) and right (c) sides, respectively. d–f, Power distribution at the fibre’s end with different diameters but the same luminous core. g and h, The ratio of the intensity detected by a detector (with an area of 1 mm2 in the centre of the fibre) and the diameter of the fibre when the luminescent core transmittance is 10% (g) and 3% (h), respectively.
Extended Data Fig. 4 Characterization of NaLuF4:Tb@NaYF4 nanoscintillators exposed to megavoltage photon beams.
a, Photograph of the experimental setup. b, Radioluminescence as a function of dose rate under 6 MV and 10 MV irradiation. c, Photograph of the experimental setup with a water tank containing water approximately 11 cm thick. d, Radioluminescence as a function of dose rate under 6 MV and 10 MV irradiation with a water tank at the front. e, Measured radioluminescence intensity over time at dose rates from 0.58 to 5.76 Gy/min. f, Radioluminescence of the nanoscintillators irradiated for 5 min at dose rates of 2.88 Gy/min and 5.76 Gy/min.
Extended Data Fig. 5 Comparison of results from linear support vector machine (SVM), linear regression, and neural network (NN) regression.
a, Measured radioluminescence intensity over time at a dose of 10 mGy and dose rates from 4 to 16 mGy/min. b and c, Comparison of the accuracy of dose estimation using different algorithms and feature parameter selection approaches, respectively. d–f, Regression results using linear SVM (d), linear regression (e), and the NN regression algorithm (f), with prediction-true value scatter plots (top panels), residual plots (middle panels) and RMSE under different feature strategies (bottom panels). When radioluminescence intensity (L), afterglow intensity (A) at different times, and temperature (T) are selected as feature parameters of the regression algorithms, the RMSE of the three algorithms is 0.304, 0.141, and 0.04 mGy/min, respectively. The RMSE statistics for each algorithm are derived from 200 executions. In c–f, bar plots and error bars show the mean ± s.d.
Extended Data Fig. 6 Effect of the relative direction of the capsule and the X-rays on the radioluminescence intensity.
The test schematic (left column), the simulated power distribution of the fibre end (middle column), and the power detected by the detector (right column), with X-rays incident along the z-axis and the capsule rotating about the y-axis (a), the z-axis (b), and the x-axis (c). d. Power detected by the sensor versus angle of rotation of the capsule around the y-axis for a 50 kV or 6 MV beam. e, Average percent angular dependence of the dosimeter for different beam angles.
Extended Data Fig. 7 Synthesis and characterization of the optical pH sensor.
a, Schematic of the polyaniline synthesis. In a typical procedure, aniline liquid and ammonium persulfate (APS) were added to hydrochloric acid, followed by the addition of a PDMS substrate to the solution. b, Normalized transmission spectra of the pH-sensing film at various pH levels, with emission ranging from 300 nm to 800 nm. c, Changes in G/B colour ratio versus pH. d, pH response of various devices fabricated from the same batch of polyaniline films. e, G/B colour ratio of the transmitted afterglow through the polyaniline film at different pH levels. f, Dynamic characteristics of pH sensing with afterglow. g, G/B colour ratio as a function of time at pH 3.5. In e and g, error bars show the s.d. of colour ratios.
Extended Data Fig. 8 Circuit design and characteristics, as well as the mobile application.
a, System-level block diagram showing signal conversion, processing, and wireless transmission from sensors to user interface. To extend battery life, the system is designed to include a sleep state, a Bluetooth state, and a work state, with the Bluetooth chip in the sleep state the majority of the time. Power consumption in each state was 6.3 μW (1.9 μA, 3.3 V), 660 μW (200 μA, 3.3 V), and 9.57 mW (2.9 mA, 3.3 V). b, The battery voltage changes over time in each of the three operating modes. c, Photographs of the printed circuit boards. The circuit, which has a thickness of 200 μm and a diameter of 7 mm, is easily assembled and fits well into a standard size 2 capsule. d, Thermal images of the circuit board after various times of operation. Circuit heating was less than 0.2 °C after 30 min of operation. The scale bar is 5 mm. e, The application’s homepage prior to Bluetooth pairing. f, Real-time display of representative data from the capsule. g, Historical data analysis and display.
Extended Data Fig. 9 In vitro experimental setup, as well as dose and pH calibration and resolution testing.
a, Capsule operation in distilled water. The water tank was placed on a stirring hot plate to keep the solution temperature between 32 °C and 46 °C. The pH was measured with a commercial pH meter and varied by controlling the ratio of HCl and NaOH supplied to the tank via a microsyringe pump. b, Photograph of the experimental setup used for in vitro demonstration of the capsule. c, Photograph of the capsule operating wirelessly under a fresh ex vivo porcine bone sample. d, Calibration of the relationship between the dose delivered to the capsule and radioluminescence. The data show excellent linearity, with an average accuracy error of less than 0.5%. e, Radioluminescence intensity at different dose rates. The minimum detectable dose rate difference was 1.8 μGy/min. f, Calibration of the relationship between pH and the G/B colour ratio. The average accuracy error is approximately 0.13. g, G/B colour ratio at different pH values. The minimum detectable pH difference was 0.02, corresponding to a difference of 0.009 in sensor output colour ratio. h, Temperature change measured by the capsule from 36 °C to 38.5 °C in 0.5 °C intervals. It should be noted that the sensor needs to be calibrated before it can be used to measure absolute temperature. i, Response measurements of the capsule under different thicknesses of fresh ex vivo porcine samples at different dose rates.
Extended Data Fig. 10 Irradiation administration via the capsule.
a, Temperature change recorded by the capsule over time in a stable experimental environment. b, Change in capsule pH over time, as measured by the persistent luminescence of NaLuF4:Tb@NaYF4 nanoscintillators. c–e, Time-course results of the absorbed dose, pH, and temperature of different capsules in two rabbits. f, Correlation between dose rate and pH changes. The correlation coefficient was obtained by linear fitting.
Supplementary information
Supplementary Information
Supplementary table.
Supplementary Video 1
Characteristics of the persistent NaLuF4:Tb@NaYF4 nanoscintillators and the pH-sensing polyaniline film at different X-ray dose rates and pH.
Supplementary Video 2
Exploded 3D view of the capsule dosimeter.
Supplementary Video 3
Demonstration of the multimodal X-ray dosimeter for in situ radiation dose detection and simultaneous real-time pH and temperature monitoring.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, B., Yi, L., Hu, D. et al. A swallowable X-ray dosimeter for the real-time monitoring of radiotherapy. Nat. Biomed. Eng 7, 1242–1251 (2023). https://doi.org/10.1038/s41551-023-01024-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-023-01024-2
This article is cited by
-
A swallowable X-ray dosimeter
Nature Biomedical Engineering (2023)
-
An easy-to-swallow pill monitors X-ray dosage
Nature (2023)