Abstract
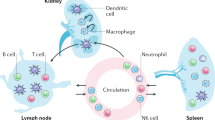
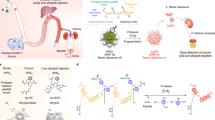
Tracking the immune microenvironment of tumours is essential for understanding the mechanisms behind the effectiveness of cancer immunotherapies. Molecular imaging of tumour-infiltrating leukocytes (TILs) can be used to non-invasively monitor the tumour immune microenvironment, but current imaging agents do not distinguish TILs from leukocytes resident in other tissues. Here we report a library of activatable molecular probes for the imaging, via near-infrared fluorescence, of specific TILs (including M1 macrophages, cytotoxic T lymphocytes and neutrophils) in vivo in real time and also via excreted urine, owing to the probes’ renal clearance. The fluorescence of the probes is activated only in the presence of both tumour and leukocyte biomarkers, which allows for the imaging of populations of specific TILs in mouse models of cancers with sensitivities and specificities similar to those achieved via flow-cytometric analyses of biopsied tumour tissues. We also show that the probes enable the non-invasive evaluation of the immunogenicity of different tumours, the dynamic monitoring of responses to immunotherapies and the accurate prediction of tumour growth under various treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding author on reasonable request. Source data are provided with this paper.
References
Mellman, I., Coukos, G. & Dranoff, G. Cancer immunotherapy comes of age. Nature 480, 480–489 (2011).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Bruni, D., Angell, H. K. & Galon, J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 20, 662–680 (2020).
Fridman, W. H., Zitvogel, L., Sautes-Fridman, C. & Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 14, 717–734 (2017).
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015).
Gutkin, D. W. & Shurin, M. R. Clinical evaluation of systemic and local immune responses in cancer: time for integration. Cancer Immunol. Immunother. 63, 45–57 (2014).
Roumanes, D., Newell, E. & Fehlings, M. Immune profiling of tumour-infiltrating T cells using mass cytometry. J. Clin. Oncol. 37, 2607–2607 (2019).
Shyamala, K., Girish, H. C. & Murgod, S. Risk of tumour cell seeding through biopsy and aspiration cytology. J. Int. Soc. Prev. Community Dent. 4, 5–11 (2014).
Santin, A. D. et al. Phenotypic and functional analysis of tumour-infiltrating lymphocytes compared with tumour-associated lymphocytes from ascitic fluid and peripheral blood lymphocytes in patients with advanced ovarian cancer. Gynecol. Obstet. Invest. 51, 254–261 (2001).
Nishino, M., Hatabu, H. & Hodi, F. S. Imaging of cancer immunotherapy: current approaches and future directions. Radiology 290, 9–22 (2019).
Seung, S. K. et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2: tumour and immunological responses. Sci. Transl Med. 4, 137ra174 (2012).
Larimer, B. M. et al. Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res. 77, 2318–2327 (2017).
Ahrens, E. T. & Bulte, J. W. M. Tracking immune cells in vivo using magnetic resonance imaging. Nat. Rev. Immunol. 13, 755–763 (2013).
Rashidian, M. et al. Predicting the response to CTLA-4 blockade by longitudinal non-invasive monitoring of CD8 T cells. J. Exp. Med. 214, 2243–2255 (2017).
Tavaré, R. et al. An effective immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res. 76, 73–82 (2016).
Cheng, P. & Pu, K. Molecular imaging and disease theranostics with renal-clearable optical agents. Nat. Rev. Mater. 6, 1095–1113 (2021).
Wu, L., Huang, J., Pu, K. & James, T. D. Dual-locked spectroscopic probes for sensing and therapy. Nat. Rev. Chem. 5, 406–421 (2021).
Huang, J., Li, J., Lyu, Y., Miao, Q. & Pu, K. Molecular optical imaging probes for early diagnosis of drug-induced acute kidney injury. Nat. Mater. 18, 1133–1143 (2019).
He, S., Li, J., Lyu, Y., Huang, J. & Pu, K. Near-Infrared fluorescent macromolecular reporters for real-time imaging and urinalysis of cancer immunotherapy. J. Am. Chem. Soc. 142, 7075–7082 (2020).
Nguyen, A. et al. Granzyme B nanoreporter for early monitoring of tumour response to immunotherapy. Sci. Adv. 6, eabc2777 (2020).
Mac, Q. D. et al. Non-invasive early detection of acute transplant rejection via nanosensors of granzyme B activity. Nat. Biomed. Eng. 3, 281–291 (2019).
Zhang, Y. et al. Activatable polymeric nanoprobe for near-infrared fluorescence and photoacoustic imaging of T lymphocytes. Angew. Chem. Int. Ed. 60, 5921–5927 (2021).
Awad, F. et al. Impact of human monocyte and macrophage polarization on NLR expression and NLRP3 inflammasome activation. PLoS ONE 12, e0175336 (2017).
Talanian, R. V. et al. Substrate specificities of caspase family proteases. J. Biol. Chem. 272, 9677–9682 (1997).
Casciola-Rosen, L. et al. Mouse and human granzyme B have distinct tetrapeptide specificities and abilities to recruit the bid pathway. J. Biol. Chem. 282, 4545–4552 (2007).
Nakajima, K., Powers, J. C., Ashe, B. M. & Zimmerman, M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J. Biol. Chem. 254, 4027–4032 (1979).
Palmieri, E. M. et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat. Commun. 11, 698 (2020).
Hu, J., Whittaker, M. R., Yu, S. H., Quinn, J. F. & Davis, T. P. Nitric oxide (NO) cleavable biomimetic thermoresponsive double hydrophilic diblock copolymer with tunable LCST. Macromolecules 48, 3817–3824 (2015).
Zheng, X. et al. Hypoxia-specific ultrasensitive detection of tumours and cancer cells in vivo. Nat. Commun. 6, 5834 (2015).
Pasqualini, R. et al. Aminopeptidase N is a receptor for tumour-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 60, 722–727 (2000).
Nourshargh, S. & Alon, R. Leukocyte migration into inflamed tissues. Immunity 41, 694–707 (2014).
Ning, X. et al. Maltodextrin-based imaging probes detect bacteria in vivo with high sensitivity and specificity. Nat. Mater. 10, 602–607 (2011).
Shim, J. S. et al. Irreversible inhibition of CD13/aminopeptidase N by the antiangiogenic agent curcumin. Chem. Biol. 10, 695–704 (2003).
Taylor, M. A. et al. Longitudinal immune characterization of syngeneic tumour models to enable model selection for immune oncology drug discovery. J. Immunother. Cancer 7, 328 (2019).
Zitvogel, L., Apetoh, L., Ghiringhelli, F. & Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 8, 59–73 (2008).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Van den Berg, T. K. & Valerius, T. Myeloid immune-checkpoint inhibition enters the clinical stage. Nature Rev. Clin. Oncol. 16, 275–276 (2019).
Edin, S. et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS ONE 7, e47045 (2012).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumours predict clinical outcome. Science 313, 1960–1964 (2006).
Kaneko, M. et al. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in advanced colorectal cancer patients receiving oxaliplatin-based chemotherapy. Oncology 82, 261–268 (2012).
Mosely, S. I. et al. Rational selection of syngeneic preclinical tumour models for immunotherapeutic drug discovery. Cancer Immunol. Res. 5, 29–41 (2017).
Widen, J. C. et al. AND-gate contrast agents for enhanced fluorescence-guided surgery. Nat. Biomed. Eng. 5, 264–277 (2021).
Fernandez, A., Thompson, E. J., Pollard, J. W., Kitamura, T. & Vendrell, M. A fluorescent activatable AND-gate chemokine CCL2 enables in vivo detection of metastasis-associated macrophages. Angew. Chem. Int. Ed. 58, 16894–16898 (2019).
Wang, W. et al. An “AND”-logic-gate-based fluorescent probe with dual reactive sites for monitoring extracellular methylglyoxal level changes of activated macrophages. Chem. Commun. 57, 8166–8169 (2021).
Madaan, A., Verma, R., Singh, A. T., Jain, S. K. & Jaggi, M. A stepwise procedure for isolation of murine bone marrow and generation of dendritic cells. J. Biol. Methods 1, e1 (2014).
Ohms, M., Möller, S. & Laskay, T. An attempt to polarize human neutrophils toward N1 and N2 phenotypes in vitro. Front. Immunol. 11, 532 (2020).
Acknowledgements
K.P. thanks the Singapore Ministry of Education, Academic Research Fund Tier 1 (RG125/19; RT05/20), Academic Research Fund Tier 2 (MOE2018-T2-2-042; MOE-T2EP30220-0010) and the Singapore National Research Foundation (NRF-NRFI07-2021-0005) for financial support.
Author information
Authors and Affiliations
Contributions
K.P. and S.H. conceived and designed the study. S.H. performed the probe-synthesis experiments and the in vitro, in vivo and ex vivo experiments. S.H. and P.C. performed whole-slide imaging experiments. K.P. and S.H. contributed to the analysis and interpretation of the results and to the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Yingxiao Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Correlation of R-NIRFs with their respective lymphocytes against CT26 tumours.
a–c, absolute number of TILs per million living cells of CT26 tumours after different treatments. Absolute number of (a) M1 macrophages, (b) CTLs and (c) neutrophils per million living cells. aPD-L1 versus saline, M1 macrophages: P = 0.0182; CTLs: P = 0.0143; neutrophils: P = 0.0034. aCD47 versus saline, M1 macrophages: P = 0.0017; CTLs: P = 0.0189; neutrophils: P = 0.0050. aPD-L1/aCD47 versus saline, M1 macrophages: P = 0.0002; CTLs: P = 0.0046; neutrophils: P = 0.0012. d–f, absolute number of biomarker (enzyme) positive non-target and target cells. d, Absolute number of iNOS−Cas-1+ and iNOS+Cas-1+ cells per million living cells. iNOS−Cas-1+ versus iNOS+Cas-1+ cells, saline: P < 0.00001; aPD-L1: P < 0.00001; aCD47: P < 0.00001; aPD-L1/ aCD47: P < 0.00001. e, Absolute number of CD3−GrB+ and CD3+CD8+GrB+ cells per million living cells. CD3−GrB+ versus CD3+CD8+GrB+ cells, saline: P = 0.0073; aPD-L1: P = 0.0001; aCD47: P = 0.0291; aPD-L1/ aCD47: P < 0.00001. f, Absolute number of Ly-6G−NE+ and Ly-6G+NE+ cells per million living cells. Ly-6G−NE+ versus Ly-6G+NE+ cells, saline: P = 0.0003; aPD-L1: P = 0.0029; aCD47: P = 0.0002; aPD-L1/ aCD47: P = 0.0002. g–i, Correlation between numbers of TILs and R-NIRFs of TAMPs in the tumour regions of CT26 tumour-bearing mice after different treatments. Correlation between (g) number of M1 macrophages per million living cells and R-NIRFM1, (h) number of CTLs per million living cells and R-NIRFCTL, and (i) number of neutrophils per million living cells and R-NIRFNE.
Extended Data Fig. 2 Antitumour efficacy.
Tumour growth curves (n = 6) of (a) 4T1 tumour-bearing mice and (b) CT26 tumour-bearing mice. For 4T1 tumour-bearing mice: aPD-L1 versus saline: P = 0.0005; Oxa versus saline: P < 0.0001; aPD-L1/Oxa versus saline: P < 0.0001. For CT26 tumour-bearing mice: aPD-L1 versus saline: P < 0.0001; aCD47 versus saline: P = 0.0002; aPD-L1/aCD47 versus saline: P < 0.0001. Body weights curves of (c) 4T1 tumour-bearing mice and (d) CT26 tumour-bearing mice. Overall survival curves (n = 6) of (e) 4T1 tumour-bearing mice and (f) CT26 tumour-bearing mice. For 4T1 tumour-bearing mice, aPD-L1/Oxa versus saline: P = 0.0008. For CT26 tumour-bearing mice, aPD-L1/aCD47 versus saline: P = 0.0005. 4T1 tumour-bearing mice were intraperitoneally injected with saline, Oxa (6 mg/kg), aPD-L1 (10 mg/kg) and aPD-L1/Oxa at day 0, 2 and 4. CT26 tumour-bearing mice were intraperitoneally injected with saline, aCD47 (10 mg/kg), aPD-L1 (10 mg/kg) and aPD-L1/aCD47 at day 0, 2 and 4.
Extended Data Fig. 3 Correlation between R-NIRFs and tumours.
a, Correlation between urinary R-NIRFs of TAMPs and in vivo R-NIRFs of TAMPs in tumour regions of tumour-bearing mice with different treatments. b, Correlation between TILs and urinary R-NIRFs of TAMPs of tumour-bearing mice with different treatments.
Supplementary information
Supplementary Information
Supplementary figures, tables and datasets.
Source data
Source data for Fig. 2.
Source data.
Source data for Fig. 3.
Source data.
Source data for Fig. 4
Source data.
Source data for Fig. 5
Source data.
Source data for Fig. 6.
Source data.
Source data for Extended Data Fig. 1.
Source data.
Source data for Extended Data Fig. 2.
Source data.
Source data for Extended Data Fig. 3.
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, S., Cheng, P. & Pu, K. Activatable near-infrared probes for the detection of specific populations of tumour-infiltrating leukocytes in vivo and in urine. Nat. Biomed. Eng 7, 281–297 (2023). https://doi.org/10.1038/s41551-023-01009-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-023-01009-1
This article is cited by
-
Artificial urinary biomarker probes for diagnosis
Nature Reviews Bioengineering (2024)
-
Rational design of biodegradable semiconducting polymer nanoparticles for NIR-II fluorescence imaging-guided photodynamic therapy
Nano Research (2024)
-
Establishment of NaLuF4:15%Tb-based low dose X-PDT agent and its application on efficient antitumor therapy
International Journal of Minerals, Metallurgy and Materials (2024)
-
In vivo bioluminescence imaging of natural bacteria within deep tissues via ATP-binding cassette sugar transporter
Nature Communications (2023)