Abstract
Hepatobiliary magnetic resonance imaging (MRI) can inform the diagnosis of liver tumours in patients with liver cirrhosis and hepatitis. However, its clinical utility has been hampered by the lack of sensitive and specific contrast agents, partly because hepatocyte-specific nanoparticles, regardless of their surface ligands, are readily sequestered by Kupffer cells. Here we show, in rabbits, pigs and macaques, that the performance of hepatobiliary MRI can be enhanced by an ultrasmall nanoparticle composed of a manganese ferrite core (3 nm in diameter) and poly(ethylene glycol)-ethoxy-benzyl surface ligands binding to hepatocyte-specific transmembrane metal and anion transporters. The nanoparticle facilitated faster, more sensitive and higher-resolution hepatobiliary MRI than the clinically used contrast agent gadoxetate disodium, a substantial enhancement in the detection rate (92% versus 48%) of early-stage liver tumours in rabbits, and a more accurate assessment of biliary obstruction in macaques. The nanoparticle’s performance and biocompatibility support the further translational development of liver-specific MRI contrast agents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the paper and its Supplementary Information. The raw and analyzed datasets are too large to be readily shared publicly but can be made available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Freddie, B. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Forner, A., Reig, M. & Bruix, J. Hepatocellular carcinoma. Lancet 391, 1301–1314 (2018).
Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 380, 1450–1462 (2019).
Banales, J. M. et al. Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 13, 261–280 (2016).
Rizvi, S. et al. Cholangiocarcinoma-evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 15, 95–111 (2018).
Anwanwan, D. et al. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 1873, 188314 (2019).
Maluccio, M. & Covey, A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 62, 394–399 (2012).
Yu, M. H. et al. Small (≤1 cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology 271, 748–760 (2014).
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Hepatobiliary Cancer Version.1 (NCCN, 2018).
European Association for the Study of the Liver. EASL clinical practice guideline: management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Choi, J. Y., Lee, J. M. & Sirlin, C. B. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. development, growth, and spread: key pathologic and imaging aspects. Radiology 272, 635–654 (2014).
Choi, J. Y., Lee, J. M. & Sirlin, C. B. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 273, 30–50 (2014).
Beers, B. E. V., Pastor, C. M. & Hussain, H. K. Primovist, eovist: what to expect? J. Hepatol. 57, 421–429 (2012).
Vilgrain, V., Beers, B. E. V. & Pastor, C. M. Insights into the diagnosis of hepatocellular carcinomas with hepatobiliary MRI. J. Hepatol. 64, 708–716 (2016).
Mi, P. et al. A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nat. Nanotechnol. 11, 724–730 (2016).
Islam, M. K. et al. Manganese complex of ethylenediaminetetraacetic acid (EDTA)-benzothiazole aniline (BTA) conjugate as a potential liver-targeting MRI contrast agent. J. Med. Chem. 60, 2993–3001 (2017).
Wang, J. et al. Manganese-based contrast agents for magnetic resonance imaging of liver tumors: structure-activity relationships and lead candidate evaluation. J. Med. Chem. 61, 8811–8824 (2018).
Islam, M. K. et al. Synthesis and evaluation of manganese (II)-Based ethylenediaminetetraacetic acid-ethoxybenzyl conjugate as a highly stable hepatobiliary magnetic resonance imaging contrast agent. Bioconjug. Chem. 29, 3614–3625 (2018).
Kim, M.-J. Improving survival with gadoxetic acid-enhanced MRI for hepatocellular carcinoma. Radiology 295, 125–126 (2020).
Lee, D. H. et al. Diagnostic performance of gadoxetic acid-enhanced liver MR imaging in the detection of HCCs and allocation of transplant recipients on the basis of the Milan criteria and UNOS guidelines: correlation with histopathologic findings. Radiology 274, 149–160 (2014).
Okada, M. et al. Biochemical and clinical predictive approach and time point analysis of hepatobiliary phase liver enhancement on Gd-EOB-DTPA-enhanced MR images: a multicenter study. Radiology 281, 474–483 (2016).
Kang, T. W. et al. Use of gadoxetic acid-enhanced liver MRI and mortality in more than 30 000 patients with hepatocellular carcinoma: a nationwide analysis. Radiology 295, 114–124 (2020).
Gale, E. M. et al. A manganese-based alternative to gadolinium: contrast-enhanced MR angiography, excretion, pharmacokinetics, and metabolism. Radiology 286, 865–872 (2018).
Wahsner, J. et al. Chemistry of MRI contrast agents: current challenges and new frontiers. Chem. Rev. 119, 957–1057 (2019).
Park, S. M. et al. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2, 17014 (2017).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Tsoi, K. M. et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 15, 1212–1221 (2016).
Wang, Y. X. J. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J. Gastroenterol. 21, 13400–13402 (2015).
Lu, Y. et al. Iron oxide nanoclusters for T1 magnetic resonance imaging of non-human primates. Nat. Biomed. Eng. 1, 637–643 (2017).
Kim, B. H. et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 133, 12624–12631 (2011).
Wei, H. et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl Acad. Sci. USA 114, 2325–2330 (2017).
Zhang, H. et al. Ultrasmall ferrite nanoparticles synthesized via dynamic simultaneous thermal decomposition for high-performance and multifunctional T1 magnetic resonance imaging contrast agent. ACS Nano 11, 3614–3631 (2017).
Miao, Y. et al. Composition-tunable ultrasmall manganese ferrite nanoparticles: insights into their in vivo T1 contrast efficacy. Theranostics 9, 1764–1776 (2019).
Tuschl, K. et al. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat. Commun. 7, 11601 (2016).
Xin, Y. et al. Manganese transporter slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov. 3, 17025 (2017).
Schmidt, P. P. et al. Stability and transmetallation of the magnetic resonance contrast agent MnDPDP measured by EPR. J. Biol. Inorg. Chem. 7, 241–248 (2002).
Toft, K. G. Mangafodipir trisodium injection, a new contrast medium for magnetic resonance imaging: in vitro metabolism and protein binding studies of the active component MnDPDP in human blood. J. Pharm. Biomed. Anal. 15, 983–988 (1997). 1997.
Liu, X. L. et al. Coating engineering of MnFe2O4 nanoparticles with superhigh T2 relaxivity and efficient cellular uptake for highly sensitive magnetic resonance imaging. Adv. Mater. Interfaces 1, 1300069 (2014).
Kim, T. et al. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T1 contrast agents for labeling and MRI tracking of adipose-derived mesenchymal stem cells. J. Am. Chem. Soc. 133, 2955–2961 (2011).
Martinez-Finley, E. J. et al. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic. Biol. Med. 62, 65–75 (2013).
O’Neal, S. L. & Zheng, W. Manganese toxicity upon overexposure: a decade in review. Curr. Environ. Health Rep. 2, 315–328 (2015).
Semelka, R. C. & Helmberger, T. K. G. Contrast agents for MR imaging of the liver. Radiology 218, 27–38 (2001).
Reimer, P., Schneider, G. & Schima, W. Hepatobiliary contrast agents for contrast-enhanced MRI of the liver: properties, clinical development and applications. Eur. Radiol. 14, 559–578 (2004).
Ketkar-Atre, A. et al. In vivo hepatocyte MR imaging using lactose functionalized magnetoliposomes. Biomaterials 35, 1015–1024 (2014).
Kamruzzaman Selim, K. M. et al. Surface modification of magnetite nanoparticles using lactobionic acid and their interaction with hepatocytes. Biomaterials 28, 710–716 (2007).
Piché, D. et al. Targeted T1 magnetic resonance imaging contrast enhancement with extraordinarily small CoFe2O4 nanoparticles. ACS Appl. Mater. Interfaces 11, 6724–6740 (2019).
Jenkitkasemwong, S. et al. SLC39A14 deficiency alters manganese homeostasis and excretion resulting in brain manganese accumulation and motor deficits in mice. Proc. Natl Acad. Sci. USA 115, E1769–E1778 (2018).
Scheiber, I. F., Wu, Y., Morgan, S. E. & Zhao, N. The intestinal metal transporter ZIP14 maintains systemic manganese homeostasis. J. Biol. Chem. 294, 9147–9160 (2019).
Poon, W. et al. Elimination pathways of nanoparticles. ACS Nano 13, 5785–5798 (2019).
Srinivasarao, M. & Low, P. S. Ligand-targeted drug delivery. Chem. Rev. 117, 12133–12164 (2017).
Bilgin, M. et al. Diagnostic value of dynamic contrast-enhanced magnetic resonance imaging in the evaluation of the biliary obstruction. Sci. World J. 2012, 731089 (2012).
Reiner, C. S. et al. MRI assessment of biliary ductal obstruction: is there added value of T1-weighted gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MR cholangiography? AJR Am. J. Roentgenol. 201, W49–W56 (2013).
Saad, W. E. A. et al. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J. Vasc. Interv. Radiol. 21, 789–795 (2010).
Kapoor, B. S., Mauri, G. & Lorenz, J. M. Management of biliary strictures: state-of-the-art review. Radiology 289, 590–603 (2018).
Freeman, M. L. & Guda, N. M. ERCP cannulation: a review of reported techniques. Gastrointest. Endosc. 61, 112–125 (2005).
Adamek, H. E. et al. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet 356, 190–193 (2000).
Ye, L. et al. A pilot study in non-human primates shows no adverse response to intravenous injection of quantum dots. Nat. Nanotechnol. 7, 453–458 (2012).
Chiarelli, P. A. et al. Nanoparticle biokinetics in mice and nonhuman primates. ACS Nano 11, 9514–9524 (2017).
Wang, Y. X. J. Superparamagnetic iron oxide based MRI contrast agents: current status of clinical application. Quant. Imaging Med. Surg. 1, 35–40 (2011).
Kim, J. E., Kim, S. H., Lee, S. J. & Rhim, H. Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. AJR Am. J. Roentgenol. 196, 758–765 (2011).
Faletti, R. et al. Multiparametric Gd-EOB-DTPA magnetic resonance in diagnosis of HCC: dynamic study, hepatobiliary phase, and diffusion-weighted imaging compared to histology after orthotopic liver transplantation. Abdom. Imaging 40, 46–55 (2015).
Zeng, J. et al. Anchoring group effects of surface ligands on magnetic properties of Fe3O4 nanoparticles: towards high performance MRI contrast agents. Adv. Mater. 26, 2694–2698 (2014).
Bize, P. et al. Antitumoral effect of sunitinib-eluting beads in the rabbit VX2 tumor model. Radiology 280, 425–435 (2016).
Chang, J. M. et al. Dynamic contrast-enhanced magnetic resonance imaging evaluation of VX2 carcinoma in a rabbit model. Invest. Radiol. 45, 655–661 (2010).
Shao, H. et al. Diffusion-weighted MR imaging allows monitoring the effect of combretastatin A4 phosphate on rabbit implanted VX2 tumor model: 12-day dynamic results. Eur. J. Radiol. 81, 578–583 (2012).
Acknowledgements
We acknowledge the financial support provided by the National Key R&D Program of China (2021YFA1201401 to H.F.), the National Natural Science Foundation of China (82150301 to H.F., 81971586 to Y.G., 81871417 to J.J., 82072063 to X.L., 22072115 to M.P. and 31901003 to X.L.), the Shaanxi Province Funds for Distinguished Young Scholars (2019JC-27 to H.F.), the Sichuan Science and Technology Program (2020YFS0050 to Y.G.), the Guangdong Natural Science Foundation (2018A030313919 to J.J.), the Shaanxi Natural Science Foundation (2021JQ-459 to H.Z.) and the China Postdoctoral Science Foundation (2019M653719 to H.Z.). We also acknowledge A. Li (Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry and Materials Science, Northwest University) for assistance in the theoretical calculations and helpful discussion.
Author information
Authors and Affiliations
Contributions
H.F., H.Z., Y.G. and J.J. conceived and designed the experiments. H.Z., Y.Q., Y.M., Z.L., C.X., L.L., J.C., K.X. and S.S. performed the experiments. H.F., H.Z., Y.H., X.L., C.Z., Y.Y., M.P. and Y.W. analyzed the results. H.Z., Y.G., J.J., Y.H., B.-H.B. and H.F. wrote the manuscript. H.F. supervised the entire project. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
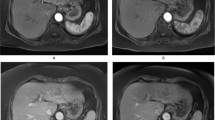
Extended Data Fig. 1 Comparison of MnFe2O4-EOB-PEG and Gd-EOB-DTPA for the detection of small liver tumours.
a, Representative MnFe2O4-EOB-PEG and Gd-EOB-DTPA enhanced MRI of rabbit’s liver using the starvibe sequence. One rabbit was randomly implanted 5 pieces of VX2 tumours in liver. All five tumours were detected using the MnFe2O4-EOB-PEG, the tumour 4 and tumour 5 were absent on the Gd-EOB-DTPA-enhanced MRI. b, Histopathological detection of the liver VX2 tumours with sizes of 3.72 mm × 2.47 mm (tumour 1), 3.62 mm × 3.29 mm (tumour 2), 3.88 mm × 2.17 mm (tumour 3), 2.35 mm × 2.60 mm (tumour 4) and 3.13 mm × 1.82 mm (tumour 5), respectively. Scale bar, 1 mm.
Extended Data Fig. 2
Dynamic liver MRI using MnFe2O4-EOB-PEG and gadoxetate disodium in macaque monkeys.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Supplementary Video 1
Dynamic time-resolved magnetic resonance angiography in a rabbit using an angio-twist sequence after bolus injection of MnFe2O4-EOB-PEG at a dose of 3 mg (Fe+Mn) kg−1 body weight.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Guo, Y., Jiao, J. et al. A hepatocyte-targeting nanoparticle for enhanced hepatobiliary magnetic resonance imaging. Nat. Biomed. Eng 7, 221–235 (2023). https://doi.org/10.1038/s41551-022-00975-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00975-2
This article is cited by
-
Nitric oxide nano-reactor DNMF/PLGA enables tumor vascular microenvironment and chemo-hyperthermia synergetic therapy
Journal of Nanobiotechnology (2024)
-
Zn-Shik-PEG nanoparticles alleviate inflammation and multi-organ damage in sepsis
Journal of Nanobiotechnology (2023)
-
Advances in magnetic nanoparticle-based magnetic resonance imaging contrast agents
Nano Research (2023)