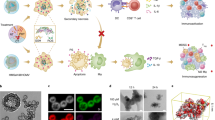
Abstract
The effectivity of cancer immunotherapies is hindered by immunosuppressive tumour microenvironments that are poorly infiltrated by effector T cells and natural killer cells. In infection and autoimmune disease, the recruitment and activation of effector immune cells is coordinated by pro-inflammatory T helper 17 (TH17) cells. Here we show that pathogen-mimicking hollow nanoparticles displaying mannan (a polysaccharide that activates TH17 cells in microbial cell walls) limit the fraction of regulatory T cells and induce TH17-cell-mediated anti-tumour responses. The nanoparticles activate the pattern-recognition receptor Dectin-2 and Toll-like receptor 4 in dendritic cells, and promote the differentiation of CD4+ T cells into the TH17 phenotype. In mice, intra-tumoural administration of the nanoparticles decreased the fraction of regulatory T cells in the tumour while markedly increasing the fractions of TH17 cells (and the levels of TH17-cell-associated cytokines), CD8+ T cells, natural killer cells and M1-like macrophages. The anti-tumoural activity of the effector cells was amplified by an agonistic antibody against the co-stimulatory receptor OX40 in multiple mouse models. Nanomaterials that induce TH17-cell-mediated immune responses may have therapeutic potential.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data are provided with this paper. All data generated in this study are available from the corresponding authors on reasonable request.
References
Medzhitov, R. & Janeway, C. Jr Innate immunity. N. Engl. J. Med 343, 338–344 (2000).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Mogensen, T. H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22, 240–273 (2009).
Zou, W. & Restifo, N. P. TH17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 10, 248–256 (2010).
Noack, M. & Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 13, 668–677 (2014).
Khader, S. A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8, 369–377 (2007).
Bettelli, E., Korn, T., Oukka, M. & Kuchroo, V. K. Induction and effector functions of TH17 cells. Nature 453, 1051–1057 (2008).
Quintana, F. J. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Solt, L. A. et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472, 491–494 (2011).
Esplugues, E. et al. Control of TH17 cells occurs in the small intestine. Nature 475, 514–518 (2011).
Xu, T. et al. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature 548, 228–233 (2017).
Hang, S. et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148 (2019).
Wilke, C. M., Bishop, K., Fox, D. & Zou, W. Deciphering the role of Th17 cells in human disease. Trends Immunol. 32, 603–611 (2011).
Knochelmann, H. M. et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 15, 458–469 (2018).
Martin-Orozco, N. et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31, 787–798 (2009).
Kryczek, I. et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114, 1141–1149 (2009).
Xie, Y. et al. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J. Exp. Med 207, 651–667 (2010).
Muranski, P. et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 35, 972–985 (2011).
Viaud, S. et al. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res. 71, 661–665 (2011).
Si, Y. et al. Adjuvant-free nanofiber vaccine induces in situ lung dendritic cell activation and TH17 responses. Sci. Adv. 6, eaba0995 (2020).
Robinson, M. J. et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med. 206, 2037–2051 (2009).
Saijo, S. et al. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691 (2010).
Netea, M. G. et al. Variable recognition of Candida albicans strains by TLR4 and lectin recognition receptors. Med Mycol. 48, 897–903 (2010).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373 (2010).
Garcia-Rubio, R., de Oliveira, H. C., Rivera, J. & Trevijano-Contador, N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 10, 2993 (2019).
Son, S. et al. Sugar-nanocapsules imprinted with microbial molecular patterns for mRNA vaccination. Nano Lett. 20, 1499–1509 (2020).
Pradhan, A. et al. Non-canonical signalling mediates changes in fungal cell wall PAMPs that drive immune evasion. Nat. Commun. 10, 5315 (2019).
Graus, M. S. et al. Mannan molecular substructures control nanoscale glucan exposure in Candida. Cell Rep. 24, 2432–2442.e2435 (2018).
Croft, M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annu. Rev. Immunol. 28, 57–78 (2010).
Aspeslagh, S. et al. Rationale for anti-OX40 cancer immunotherapy. Eur. J. Cancer 52, 50–66 (2016).
Korolenko, T. A., Bgatova, N. P. & Vetvicka, V. Glucan and mannan—two peas in a pod. Int J. Mol. Sci. 20, 3189 (2019).
Vendele, I. et al. Mannan detecting C-type lectin receptor probes recognise immune epitopes with diverse chemical, spatial and phylogenetic heterogeneity in fungal cell walls. PLoS Pathog. 16, e1007927 (2020).
Hou, Y. et al. Co-delivery of antigen and dual adjuvants by aluminum hydroxide nanoparticles for enhanced immune responses. J. Control. Release 326, 120–130 (2020).
Rennick, J. J., Johnston, A. P. & Parton, R. G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276 (2021).
Chen, C. & Gao, F.-H. Th17 cells paradoxical roles in melanoma and potential application in immunotherapy. Front. Immunol. 10, 187 (2019).
Ankathatti Munegowda, M., Deng, Y., Mulligan, S. J. & Xiang, J. Th17 and Th17-stimulated CD8+ T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunol. Immunother. 60, 1473–1484 (2011).
Shortman, K. & Heath, W. R. The CD8+ dendritic cell subset. Immunological Rev. 234, 18–31 (2010).
Cerovic, V. et al. Lymph-borne CD8α+ dendritic cells are uniquely able to cross-prime CD8+ T cells with antigen acquired from intestinal epithelial cells. Mucosal Immunol. 8, 38–48 (2015).
McGeachy, M. J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 (2009).
Bromley, S. K., Mempel, T. R. & Luster, A. D. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat. Immunol. 9, 970–980 (2008).
Xin, L. et al. Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7-1 and B7-2. Proc. Natl Acad. Sci. USA 111, 10672–10677 (2014).
Huang, A. Y. et al. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc. Natl Acad. Sci. USA 93, 9730–9735 (1996).
Kreiter, S. et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015).
Sun, X. et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat. Nanotechnol. 16, 1260–1270 (2021).
Zhao, H. & Heindel, N. D. Determination of degree of substitution of formyl groups in polyaldehyde dextran by the hydroxylamine hydrochloride method. Pharm. Res. 8, 400–402 (1991).
Lutz, M. B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999).
Acknowledgements
This work was supported in part by NIH (R01DE030691, R01DE031951, R01DK125087, R01CA271799, R01NS122536, U01CA210152 and P30CA046592), David Koch-Prostate Cancer Foundation Award in Nanotherapeutics and National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2022R1F1A1075064 and 2022R1A2C1006643). A.S.K. acknowledges financial support from the UM CBTP Training Program (NIH T32GM008353). K.S.P. acknowledges financial support from the UM TEAM Training Program (NIH T32DE007057). We thank the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC-I tetramers; and the University of Michigan Cancer Center Immunology Core for ELISA analysis.
Author information
Authors and Affiliations
Contributions
S.S., J.N. and J.J.M. designed the study. S.S., J.N., A.S.K., K.S.P., M.T.P., J.A. and B.S. performed the experiments. S.S., J.N., W.Z., S.-H.L., J.S., O.C.F. and J.J.M interpreted the data. S.S., J.N. and J.J.M. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
A patent application for particles for the delivery of biomolecules has been filed, with S.S., O.C.F. and J.J.M. as inventors. O.C.F. has financial interests in Selecta Biosciences, Tarveda Therapeutics, and Seer. J.J.M. declares financial interests for board membership, as a paid consultant, for research funding, and/or as equity holder in EVOQ Therapeutics, Saros Therapeutics and Intrinsic Medicine.
Peer review
Peer review information
Nature Biomedical Engineering thanks Miodrac Colic, Jeffrey Hubbell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
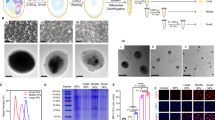
Extended Data Fig. 1 A schematic illustration of Mann-NC synthesis.
A negatively charged template silica nanoparticle was coated with branched polyethyleneimine (PEI, 25 kDa) polymer in ultra-pure water, followed by a chemical crosslinking step. The surface of PEI-coated silica nanoparticle was reacted with oxidized mannan polymers by the aldehyde-amine reaction. Then the silica nanoparticle core was selectively removed, resulting in hollow Mann-NC entirely composed of mannan polymers.
Extended Data Fig. 2 Phenotype and activity of CD4 T cells induced by Mann-NC.
(a) DCs stimulated by Mann-NC trigger the differentiation of CD4 + T-cells toward Th17 phenotype. BMDCs isolated from BDC2.5 TCR transgenic mice were treated with p31 MHC-II epitope with and without Mann-NC. After overnight incubation, p31-specific CD4 + T-cells from BDC2.5 TCR transgenic mice were added and cultured for 3 days. IL-17A released into the media was measured by ELISA, and CD4 + T-cells expressing IL-17A was measured by intracellular cytokine staining. (b) Mann-NC-inducing OX40 expression levels of Foxp3 + CD4 + T-cells and representative contour plots. (c-f) Tumor-draining lymph nodes were analysed for the frequencies of (c) CD4 + T-cells, (d) IL17 + CD4 + Th17 cells, (e) Foxp3 + CD4 + Tregs, and (f) the corresponding Th17/Treg ratio. (g) CT26 tumor-bearing mice were treated with Mann-NC as in Fig. 3a, and CD4 + T-cells in the TME on day 15 were analysed for their subsets. Data represent mean ± SEM, from a representative experiment (n = 4 (a); n = 7 (b); n = 7 for Native-Mann and Mann-NC, or 8 for PBS on day 15, or 10 for PBS on day 12 (c-f); or n = 8 (g) biologically independent samples) from two (a,b,g) or three (c-f) independent experiments. ***P < 0.001, ****P < 0.0001, analysed by one-way (a) or two-way (c-f) ANOVA with Bonferroni’s multiple comparisons test or unpaired two-tailed Student’s t-test (b, g).
Extended Data Fig. 3 Cellular uptake of Mann-NC among tumor cells and innate immune cells in local tumors.
BALB/c mice were inoculated subcutaneously with 1.5 × 105 CT26 cells on day 0 and treated by intratumoral administration of Cy5.5-Mann-NC on day 9. After 3 days, tumor tissues were analysed for Cy5.5+ cells among CD45- tumor cells, CD11c + DCs, Ly6C+ monocytes, F4/80+ macrophages, and Ly6G+ neutrophils. Data represent mean ± SEM, from a representative experiment with n = 5 biologically independent samples from two independent experiments.
Extended Data Fig. 4 Representative contour plots.
Shown are representative contour plots of IFN-γ + CD8 T-cells, granzyme B + CD8 + T-cells, IFN-γ + NK cells, and granzyme B + NK cells for the dataset shown in the main Fig. 5e-f.
Extended Data Fig. 5 Representative contour plots.
Shown are the representative contour plots of CD80 + CD11c + DCs, CD103 + CD11c + DCs, and CD8𝛼 + CD103 + CD11c + DCs for the dataset shown in the main Fig. 7a.
Extended Data Fig. 6 Immunofluorescence analysis of IL-17A expression.
Immunofluorescence images of IL-17A expression among CD4 T-cells and CD8 T-cells in TME on day 15. Scale bar represents 50 μm.
Extended Data Fig. 7 Immunofluorescence analysis of IFN-γ expression.
Immunofluorescence images of IFN-γ expression among CD4 T-cells and CD8 T-cells in TME on day 15. Scale bar represents 50 μm.
Supplementary information
Main Supplementary Information
Supplementary figures.
Source data
Source Data Figs. 3–8
Source data for tumour size.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Son, S., Nam, J., Kim, A.S. et al. Induction of T-helper-17-cell-mediated anti-tumour immunity by pathogen-mimicking polymer nanoparticles. Nat. Biomed. Eng 7, 72–84 (2023). https://doi.org/10.1038/s41551-022-00973-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00973-4
This article is cited by
-
Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy
Journal of Hematology & Oncology (2024)
-
The advances of adjuvants in mRNA vaccines
npj Vaccines (2023)
-
TH17 cells boosted by nanoparticle-bound fungal motifs
Nature Biomedical Engineering (2022)