Abstract
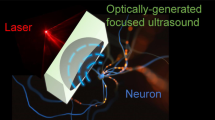
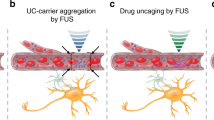
Deep brain stimulation via implanted electrodes can alleviate neuronal disorders. However, its applicability is constrained by side effects resulting from the insertion of electrodes into the brain. Here, we show that systemically administered piezoelectric nanoparticles producing nitric oxide and generating direct current under high-intensity focused ultrasound can be used to stimulate deep tissue in the brain. The release of nitric oxide temporarily disrupted tight junctions in the blood–brain barrier, allowing for the accumulation of the nanoparticles into brain parenchyma, and the piezoelectrically induced output current stimulated the release of dopamine by dopaminergic neuron-like cells. In a mouse model of Parkinson’s disease, the ultrasound-responsive nanoparticles alleviated the symptoms of the disease without causing overt toxicity. The strategy may inspire the development of other minimally invasive therapies for neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data for the figures are provided with this paper.
References
Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 539, 180–186 (2016).
Kringelbach, M. L., Jenkinson, N., Owen, S. L. & Aziz, T. Z. Translational principles of deep brain stimulation. Nat. Rev. Neurosci. 8, 623–635 (2007).
Chen, R., Canales, A. & Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2, 16093 (2017).
Chen, R., Romero, G., Christiansen, M. G., Mohr, A. & Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 347, 1477–1480 (2015).
Guduru, R. et al. Magnetoelectric ‘spin’ on stimulating the brain. Nanomedicine 10, 2051–2061 (2015).
Chen, S. et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 359, 679–684 (2018).
Rivnay, J., Wang, H., Fenno, L., Deisseroth, K. & Malliaras, G. G. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 3, e1601649 (2017).
Tu, J. et al. Controllable in vivo hyperthermia effect induced by pulsed high intensity focused ultrasound with low duty cycles. Appl. Phys. Lett. 101, 124102 (2012).
Hinchet, R. et al. Transcutaneous ultrasound energy harvesting using capacitive triboelectric technology. Science 365, 491–494 (2019).
Mace, E. et al. Functional ultrasound imaging of the brain. Nat. Methods 8, 662–664 (2011).
Kang, Y. et al. Tumor vasodilation by N-heterocyclic carbene-based nitric oxide delivery triggered by high-intensity focused ultrasound and enhanced drug homing to tumor sites for anti-cancer therapy. Biomaterials 217, 119297 (2019).
Marino, A. et al. Piezoelectric nanoparticle-assisted wireless neuronal stimulation. ACS Nano 9, 7678–7689 (2015).
Rojas, C. et al. Acoustic stimulation can induce a selective neural network response mediated by piezoelectric nanoparticles. J. Neural Eng. 15, 036016 (2018).
Hendricks, B. K., Cohen-Gadol, A. A. & Miller, J. C. Novel delivery methods bypassing the blood–brain and blood–tumor barriers. Neurosurg. Focus 38, E10 (2015).
Wiley, D. T., Webster, P., Gale, A. & Davis, M. E. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc. Natl Acad. Sci. USA 110, 8662–8667 (2013).
Ting, C. Y. et al. Concurrent blood–brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 33, 704–712 (2012).
Martinez, H. R. et al. Stem-cell transplantation into the frontal motor cortex in amyotrophic lateral sclerosis patients. Cytotherapy 11, 26–34 (2009).
Lipsman, N. et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 9, 2336 (2018).
Wong, K. H. et al. Review of current strategies for delivering Alzheimer’s disease drugs across the blood–brain barrier. Int. J. Mol. Sci. 20, 381 (2019).
Kim, T., Suh, J. & Kim, W. J. Polymeric aggregate-embodied hybrid nitric-oxide-scavenging and sequential drug-releasing hydrogel for combinatorial treatment of rheumatoid arthritis. Adv. Mater. 33, 2008793 (2021).
Parathath, S. R., Parathath, S. & Tsirka, S. E. Nitric oxide mediates neurodegeneration and breakdown of the blood–brain barrier in tPA-dependent excitotoxic injury in mice. J. Cell Sci. 119, 339–349 (2006).
Jin, Z. K. et al. MRI-guided and ultrasound-triggered release of NO by advanced nanomedicine. Nanoscale 9, 3637–3645 (2017).
Hong, S. et al. Attenuation of the in vivo toxicity of biomaterials by polydopamine surface modification. Nanomedicine 6, 793–801 (2011).
Xie, C. M. et al. Electroresponsive and cell-affinitive polydopamine/polypyrrole composite microcapsules with a dual-function of on-demand drug delivery and cell stimulation for electrical therapy. NPG Asia Mater. 9, e358 (2017).
Wei, G., Yang, G., Wei, B., Wang, Y. & Zhou, S. Near-infrared light switching nitric oxide nanoemitter for triple-combination therapy of multidrug resistant cancer. Acta Biomater. 100, 365–377 (2019).
Li, Y. et al. Antifouling, high-flux nanofiltration membranes enabled by dual functional polydopamine. ACS Appl. Mater. Interfaces 6, 5548–5557 (2014).
Herrington, T. M., Cheng, J. J. & Eskandar, E. N. Mechanisms of deep brain stimulation. J. Neurophysiol. 115, 19–38 (2016).
Neher, E. & Sakaba, T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59, 861–872 (2008).
Xicoy, H., Wieringa, B. & Martens, G. J. The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol. Neurodegener. 12, 10 (2017).
Lonart, G., Cassels, K. L. & Johnson, K. M. Nitric oxide induces calcium-dependent [3H]dopamine release from striatal slices. J. Neurosci. Res. 35, 192–198 (1993).
Rodriguez, A., Tatter, S. B. & Debinski, W. Neurosurgical techniques for disruption of the blood–brain barrier for glioblastoma treatment. Pharmaceutics 7, 175–187 (2015).
Ridnour, L. A. et al. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc. Natl Acad. Sci. USA 104, 16898–16903 (2007).
O’Sullivan, S., Medina, C., Ledwidge, M., Radomski, M. W. & Gilmer, J. F. Nitric oxide–matrix metaloproteinase-9 interactions: biological and pharmacological significance—NO and MMP-9 interactions. Biochim. Piophys. Acta 1843, 603–617 (2014).
Rempe, R. G. et al. Matrix metalloproteinase-mediated blood–brain barrier dysfunction in epilepsy. J. Neurosci. 38, 4301–4315 (2018).
Gu, Z. et al. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 297, 1186–1190 (2002).
Obermeier, B., Daneman, R. & Ransohoff, R. M. Development, maintenance and disruption of the blood–brain barrier. Nat. Med. 19, 1584–1596 (2013).
Surmeier, D. J. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 285, 3657–3668 (2018).
Kwon, O. B. et al. Dopamine regulation of amygdala inhibitory circuits for expression of learned fear. Neuron 88, 378–389 (2015).
Lippert, R. N. et al. Time-dependent assessment of stimulus-evoked regional dopamine release. Nat. Commun. 10, 336 (2019).
Williams, N. R. & Okun, M. S. Deep brain stimulation (DBS) at the interface of neurology and psychiatry. J. Clin. Invest. 123, 4546–4556 (2013).
Jackson-Lewis, V. & Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2, 141–151 (2007).
McKinnon, C. et al. Deep brain stimulation: potential for neuroprotection. Ann. Clin. Transl. Neurol. 6, 174–185 (2019).
Fadok, J. P., Dickerson, T. M. K. & Palmiter, R. D. Dopamine Is necessary for cue-dependent fear conditioning. J. Neurosci. 29, 11089–11097 (2009).
Ehlerding, E. B., Chen, F. & Cai, W. Biodegradable and renal clearable inorganic nanoparticles. Adv. Sci. 3, 1500223 (2016).
Liu, J. B., Yu, M. X., Zhou, C. & Zheng, J. Renal clearable inorganic nanoparticles: a new frontier of bionanotechnology. Mater. Today 16, 477–486 (2013).
Hao, Y. N. et al. Glutathione triggered degradation of polydopamine to facilitate controlled drug release for synergic combinational cancer treatment. J. Mater. Chem. B 7, 6742–6750 (2019).
Neubrand, A., Lindner, R. & Hoffmann, P. Room-temperature solubility behavior of barium titanate in aqueous media. J. Am. Ceram. Soc. 83, 860–864 (2000).
Morishita, T. et al. Postoperative lead migration in deep brain stimulation surgery: incidence, risk factors, and clinical impact. PLoS ONE 12, e0183711 (2017).
Ahn, J. H. et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66 (2019).
Fan, J. et al. A novel self-assembled sandwich nanomedicine for NIR-responsive release of NO. Nanoscale 7, 20055–20062 (2015).
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grants (NRF-2019R1A2C2006269 and NRF-2020R1A6A1A03047902 to C.K. and NRF-2020R1A4A1019456 and NRF-2022R1A3B1077354 to W.J.K.), by the Creative Materials Discovery Program (NRF-2018M3D1A1058813 to W.J.K.), by the Korean government (Ministry of Science and ICT) and by OmniaMed Co., Ltd.
Author information
Authors and Affiliations
Contributions
T.K. and W.J.K. conceived the idea, designed the study and directed the project. T.K. performed all the experiments and analysed the data. H.J.K. assisted with the patch-clamp set-up and with mouse-behaviour monitoring. W.C. and Jeesu Kim helped with HIFU treatment in vivo. Y.M.L. and J.H.P. assisted with the in vivo and ex vivo experiments. J.L. helped with the in vitro fluorescence imaging. C.K., J.-H.K. and Jihoon Kim provided insightful advice. T.K., Jihoon Kim and W.J.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
W.J.K. is the Chief Executive Officer at OmniaMed. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Thomas McHugh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, tables, methods and references.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, T., Kim, H.J., Choi, W. et al. Deep brain stimulation by blood–brain-barrier-crossing piezoelectric nanoparticles generating current and nitric oxide under focused ultrasound. Nat. Biomed. Eng 7, 149–163 (2023). https://doi.org/10.1038/s41551-022-00965-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00965-4
This article is cited by
-
Ultrasound-assisted tissue engineering
Nature Reviews Bioengineering (2024)
-
Designing transparent piezoelectric metasurfaces for adaptive optics
Nature Communications (2024)
-
Janus microparticles-based targeted and spatially-controlled piezoelectric neural stimulation via low-intensity focused ultrasound
Nature Communications (2024)
-
Exploiting sound for emerging applications of extracellular vesicles
Nano Research (2024)
-
The marriage of immunomodulatory, angiogenic, and osteogenic capabilities in a piezoelectric hydrogel tissue engineering scaffold for military medicine
Military Medical Research (2023)