Abstract
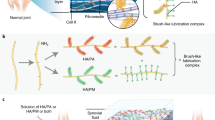
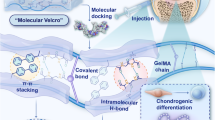
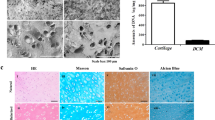
Treatments for osteoarthritis would benefit from the enhanced visualization of injured articular cartilage and from the targeted delivery of disease-modifying drugs to it. Here, by using ex vivo human osteoarthritic cartilage and live rats and minipigs with induced osteoarthritis, we report the application of collagen-binding peptides, identified via phage display, that are home to osteoarthritic cartilage and that can be detected via magnetic resonance imaging when conjugated with a superparamagnetic iron oxide. Compared with the use of peptides with a scrambled sequence, hyaluronic acid conjugated with the collagen-binding peptides displayed enhanced retention in osteoarthritic cartilage and better lubricated human osteoarthritic tissue ex vivo. Mesenchymal stromal cells encapsulated in the modified hyaluronic acid and injected intra-articularly in rats showed enhanced homing to osteoarthritic tissue and improved its regeneration. Molecular docking revealed WXPXW as the consensus motif that binds to the α1 chain of collagen type XII. Peptides that specifically bind to osteoarthritic tissue may aid the diagnosis and treatment of osteoarthritic joints.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Goldring, S. R. & Goldring, M. B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 12, 632–644 (2016).
Martel-Pelletier, J. et al. Osteoarthritis. Nat. Rev. Dis. Prim. 2, 16072 (2016).
Peat, G., McCarney, R. & Croft, P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann. Rheum. Dis. 60, 91–97 (2001).
Han, S. B., Seo, I. W. & Shin, Y. S. Intra-articular injections of hyaluronic acid or steroids associated with better outcomes than platelet-rich plasma, adipose mesenchymal stromal cells, or placebo in knee osteoarthritis: a network meta-analysis. Arthroscopy 37, 292–306 (2021).
Jones, I. A., Togashi, R., Wilson, M. L., Heckmann, N. & Vangsness, C. T. Jr. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 15, 77–90 (2019).
Brittberg, M. et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 331, 889–895 (1994).
Wakitani, S. et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J. Tissue Eng. Regen. Med. 5, 146–150 (2011).
Wei, C. C., Lin, A. B. & Hung, S. C. Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: bench, bedside, and industry. Cell Transplant. 23, 505–512 (2014).
Yubo, M. et al. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: a meta-analysis. PLoS ONE 12, e0175449 (2017).
Jo, C. H. et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am. J. Sports Med 45, 2774–2783 (2017).
Chiang, E. R. et al. Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits. PLoS ONE 11, e0149835 (2016).
Murphy, J. M., Fink, D. J., Hunziker, E. B. & Barry, F. P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 48, 3464–3474 (2003).
Kamei, G. et al. Articular cartilage repair with magnetic mesenchymal stem cells. Am. J. Sports Med 41, 1255–1264 (2013).
McHugh, J. A drug delivery system with sting. Nat. Rev. Rheumatol. 16, 248 (2020).
Cheung, C. S., Lui, J. C. & Baron, J. Identification of chondrocyte-binding peptides by phage display. J. Orthop. Res 31, 1053–1058 (2013).
Rothenfluh, D. A., Bermudez, H., O’Neil, C. P. & Hubbell, J. A. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat. Mater. 7, 248–254 (2008).
Chen, W. H. et al. In vitro stage-specific chondrogenesis of mesenchymal stem cells committed to chondrocytes. Arthritis Rheum. 60, 450–459 (2009).
Charafe-Jauffret, E. et al. Immunophenotypic analysis of inflammatory breast cancers: identification of an ‘inflammatory signature’. J. Pathol. 202, 265–273 (2004).
Stockwell, R. A. Changes in the acid glycosaminoglycan content of the matrix of ageing human articular cartilage. Ann. Rheum. Dis. 29, 509–515 (1970).
Deng, M. W. et al. Cell therapy with G-CSF-mobilized stem cells in a rat osteoarthritis model. Cell Transplant. 24, 1085–1096 (2015).
Singh, A. et al. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater. 13, 988–995 (2014).
Wu, S. C., Chang, J. K., Wang, C. K., Wang, G. J. & Ho, M. L. Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment. Biomaterials 31, 631–640 (2010).
Chung, C. & Burdick, J. A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng. Part A 15, 243–254 (2009).
Johnson, K., Reynard, L. N. & Loughlin, J. Functional characterisation of the osteoarthritis susceptibility locus at chromosome 6q14.1 marked by the polymorphism rs9350591. BMC Med. Genet. 16, 81 (2015).
Karsdal, M. A. et al. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthr. Cartil. 24, 2013–2021 (2016).
Shkhyan, R. et al. Drug-induced modulation of gp130 signalling prevents articular cartilage degeneration and promotes repair. Ann. Rheum. Dis. 77, 760–769 (2018).
Johnson, K. et al. A stem cell-based approach to cartilage repair. Science 336, 717–721 (2012).
Hollander, A. P. et al. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J. Clin. Invest. 96, 2859–2869 (1995).
Sugrue, S. P. et al. Immunoidentification of type XII collagen in embryonic tissues. J. Cell Biol. 109, 939–945 (1989).
Benito, M. J., Veale, D. J., FitzGerald, O., van den Berg, W. B. & Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 64, 1263–1267 (2005).
Myers, S. L. et al. Synovial inflammation in patients with early osteoarthritis of the knee. J. Rheumatol. 17, 1662–1669 (1990).
Sellam, J. & Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635 (2010).
Deswal, J., Arya, S. K., Raj, A. & Bhatti, A. A case of bilateral corneal perforation in a patient with severe dry eye. J. Clin. Diagn. Res 11, ND01–ND02 (2017).
Rustenburg, C. M. E. et al. Osteoarthritis and intervertebral disc degeneration: quite different, quite similar. JOR Spine 1, e1033 (2018).
Roberts, C. R. & Pare, P. D. Composition changes in human tracheal cartilage in growth and aging, including changes in proteoglycan structure. Am. J. Physiol. 261, L92–L101 (1991).
Wessel, H. et al. Type XII collagen contributes to diversities in human corneal and limbal extracellular matrices. Invest. Ophthalmol. Vis. Sci. 38, 2408–2422 (1997).
Massoudi, D. et al. NC1 long and NC3 short splice variants of type XII collagen are overexpressed during corneal scarring. Invest. Ophthalmol. Vis. Sci. 53, 7246–7256 (2012).
Liu, N. R., Chen, G. N., Wu, S. S. & Chen, R. Distinguishing human normal or cancerous esophagus tissue ex vivo using multiphoton microscopy. J. Opt. 16, 025301 (2014).
Xu, J. et al. Multiphoton microscopy for label-free identification of intramural metastasis in human esophageal squamous cell carcinoma. Biomed. Opt. Express 8, 3360–3368 (2017).
Houle, M. A. et al. Analysis of forward and backward second harmonic generation images to probe the nanoscale structure of collagen within bone and cartilage. J. Biophotonics 8, 993–1001 (2015).
Acknowledgements
We thank C.-C. Lin and the Core Facility of the Institute of Cellular and Organismic Biology (ICOB), Academia Sinica, for their assistance with C5-24, scrambled-peptide synthesis and biotin conjugation. This work was supported by the Drug Development Center, China Medical University, from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. We thank W.-T. Juan and G.-Y. Zhuo, from the Two photon core facility, China Medical University, for their assistance. This work was financially supported by the Minister of Science and Technology (MOST 106-2321-B-039-003; 109-2321-B-039 -003; 108-2221-E-039-006-MY3; 111-2326-B-039 -001), China Medical University (Grant Nos. CMU110-S-26 and CMU110-MF-83) and China Medical University Hospital (Grant No. DMR-110-228). The funding sources had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
H.-C.W. and S.-C.H. conceptualized the study and acquired funds. C.-Y.L., Y.-L.W., Y.-J.C., C.-T.H., Y.-H.C. and L.Y.C. conducted experiments. C.-Y.L., Y.-L.W., Y.-J.C., C.-T.H., Y.-H.C., L.Y.C., D.W.H. and S.-C.H. carried out data analyses. G.-W.C. carried out computational analyses. D.W.H. carried out the MRI studies. C.-Y.L., H.-C.W. and S.-C.H. wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Jeffrey Weiss and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Biopanning of phage clones targeting OA cartilage.
The OA articular cartilage specimens of knee joints from patients who received total knee joint replacement were separated into two parts, cell lysates and square pieces, for screening a phage peptide display library to identify clones targeting OA cartilage. a, Preparation of clinical cartilage tissues for phage clones biopanning. b, Biopanning of cartilage lysate. c, Biopanning of cartilage pieces.
Supplementary information
Supplementary Information
Supplementary methods, figures, tables and references.
Supplementary data
Source data for the Supplementary figures
Source data
Source Data For Fig. 1b-c
Source data.
Source Data For Fig. 3c
Source data.
Source Data For Fig. 4b
Source data.
Source Data For Fig. 5d
Source data.
Source Data For Fig. 6c-d
Source data.
Source Data For Fig. 6b
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, CY., Wang, YL., Chen, YJ. et al. Collagen-binding peptides for the enhanced imaging, lubrication and regeneration of osteoarthritic articular cartilage. Nat. Biomed. Eng 6, 1105–1117 (2022). https://doi.org/10.1038/s41551-022-00948-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00948-5