Abstract
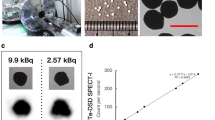
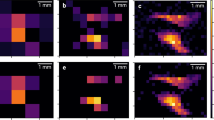
The insufficient energy and spatial resolutions of radionuclide imaging with conventional scintillation detectors restrict the visualization of multiple radionuclides and of microstructures in tissue. Here we report the development and performance of an imaging system equipped with a cadmium telluride diode detector that achieves an energy resolution of 1.7% at 140 keV and a spatial resolution of 250 μm. The combination of high-resolution spectra fitted to an X-ray analysis model of the emission lines of the radionuclides in a chosen energy band allowed us to accurately determine individual radiation activities from three radionuclides to simultaneously visualize thyroid tissue (via intravenously administered iodine-125), mandibular lymph nodes (via the intramuscular injection of indium-111) and parotid lymph nodes (via a subcutaneous injection of technetium-99m) in mice. Multi-radionuclide imaging may find advantageous applications in biomedical imaging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
Code availability
The custom code for data collection, spectral fitting and image reconstruction is intended to be used for the joint development of radionuclide imaging systems with companies. The code is, however, available for research purposes from the corresponding authors on request.
References
Jang, B. S. MicroSPECT and MicroPET imaging of small animals for drug development. Toxicol. Res 29, 1–6 (2013).
Weissleder, R. & Pittet, M. J. Imaging in the era of molecular oncology. Nature 452, 580–589 (2008).
Blow, N. In vivo molecular imaging: the inside job. Nat. Methods 6, 465–469 (2009).
Franc, B. L., Acton, P. D., Mari, C. & Hasegawa, B. H. Small-animal SPECT and SPECT/CT: important tools for preclinical investigation. J. Nucl. Med. 49, 1651–1663 (2008).
Nishimura, T. et al. Prediction of functional recovery and prognosis in patients with acute myocardial infarction by 123I-BMIPP and 201Tl myocardial single photon emission computed tomography: a multicenter trial. Ann. Nucl. Med 12, 237–248 (1998).
Yamanaka, H., Suzuki, T., Kishida, H., Nagasawa, K. & Takano, T. Relationship between the mismatch of 123I-BMIPP and 201Tl myocardial single-photon emission computed tomography and autonomic nervous system activity in patients with acute myocardial infarction. Int. Heart J. 47, 193–207 (2006).
Kawaguchi, K. et al. Quantitative estimation of infarct size by simultaneous dual radionuclide single photon emission computed tomography: comparison with peak serum creatine kinase activity. Am. Heart J. 121, 1353–1360 (1991).
Hashimoto, T. et al. Significance of technetium-99m/thallium-201 overlap on simultaneous dual emission computed tomography in acute myocardial infarction. Am. J. Cardiol. 61, 1181–1186 (1988).
Mullan, B. P. Nuclear medicine imaging of the parathyroid. Otolaryngol. Clin. North Am. 37, 909–939 (2004).
Lukas, M., Kluge, A., Beindorff, N. & Brenner, W. Multi-isotope capabilities of a small-animal multi-pinhole SPECT system. J. Nucl. Med. 61, 152–161 (2020).
Takeda, S. et al. A high-resolution CdTe imaging detector with multi-pinhole optics for in-vivo molecular imaging. Nucl. Instrum. Methods A 912, 57–60 (2018).
Takahashi, T. et al. High-resolution Schottky CdTe diode for hard X-ray and gamma-ray astronomy. Nucl. Instrum. Methods A 436, 111–119 (1999).
Takahashi, T. & Watanabe, S. Recent progress in CdTe and CdZnTe detectors. IEEE Trans. Nucl. Sci. 48, 950–959 (2001).
Katsuragawa, M. et al. Suzaku X-ray observations of the mixed-morphology supernova remnant CTB 1. Publ. Astron. Soc. Jpn 70, 110 (2018).
Matsumura, H., Tanaka, T., Uchida, H., Okon, H. & Tsuru, T. G. Toward the understanding of the physical origin of recombining plasma in the supernova remnant IC 443. Astrophys. J. 851, 73 (2017).
Kobayashi, H. et al. Multimodal nanoprobes for radionuclide and five-color near-infrared optical lymphatic imaging. ACS Nano 1, 258–264 (2007).
Tavares, M. G. et al. The use of 99m Tc-phytate for sentinel node mapping in melanoma, breast cancer and vulvar cancer: a study of 100 cases. Eur. J. Nucl. Med. 28, 1597–1604 (2001).
Riedel, C., Dohan, O., De la Vieja, A., Ginter, C. S. & Carrasco, N. Journey of the iodide transporter NIS: from its molecular identification to its clinical role in cancer. Trends Biochem. Sci. 26, 490–496 (2001).
Dadachova, E. & Carrasco, N. The Na/I symporter (NIS): imaging and therapeutic applications. Semin. Nucl. Med. 34, 23–31 (2004).
Ito, T. et al. Experimental evaluation of the GE NM/CT 870 CZT clinical SPECT system equipped with WEHR and MEHRS collimator. J. Appl. Clin. Med Phys. 22, 165–177 (2021).
Erlandsson, K., Kacperski, K., van Gramberg, D. & Hutton, B. F. Performance evaluation of D-SPECT: a novel SPECT system for nuclear cardiology. Phys. Med. Biol. 54, 2635–2649 (2009).
Matsunari, I. et al. Performance evaluation of the eXplore speCZT preclinical imaging system. Ann. Nucl. Med 28, 484–497 (2014).
Weng, F., Zan, Y., Bagchi, S., Huang, Q. & Seo, Y. Energy window optimization in dual-isotope SPECT brain imaging with 99mTc/123I via CZT-based detectors. J. Nucl. Med. 56, 1803–1803 (2015).
Laforest, R. et al. Dual nuclide quantitative imaging with 99mTc/123I using CZT gamma camera. J. Nucl. Med. 58, 742–742 (2017).
Ivashchenko, O. et al. Quarter-millimeter-resolution molecular mouse imaging with U-SPECT+. Mol. Imaging https://doi.org/10.2310/7290.2014.00053 (2015).
Lifante, J., Shen, Y., Ximendes, E., Rodríguez, E. M. & Ortgies, D. H. The role of tissue fluorescence in in vivo optical bioimaging. J. Appl. Phys. 128, 171101 (2020).
Watanabe, S. et al. High energy resolution hard X-ray and gamma-ray imagers using CdTe diode devices. IEEE Trans. Nucl. Sci. 56, 777–782 (2009).
Takahashi, T. et al. Hitomi (ASTRO-H) X-ray astronomy satellite. J. Astron. TeIesc. Instrum. Syst. 4, 021402 (2018).
Nakazawa, K. et al. Hard X-ray imager onboard Hitomi (ASTRO-H). J. Astron. Telesc. Instrum. Syst. 4, 021410 (2018).
Sato, G. et al. The Si/CdTe semiconductor camera of the ASTRO-H Hard X-ray Imager (HXI). Nucl. Instrum. Methods Phys. Res. A 831, 235–241 (2016).
Update of X Ray and Gamma Ray Decay Data Standards for Detector Calibration and Other Applications (International Atomic Energy Agency, 2007).
Lange, K. & Carson, R. EM reconstruction algorithms for emission and transmission tomography. J. Comput. Assist. Tomogr. 8, 306–316 (1984).
Brun, R. & Rademakers, F. ROOT—an object oriented data analysis framework. Nucl. Instrum. Methods Phys. Res. A 389, 81–86 (1997).
Robitaille, T. P. et al. Astropy: a community Python package for astronomy. Astron. Astrophys. 558, A33 (2013).
Price-Whelan, A. M. et al. The Astropy project: building an open-science project and status of the v2. 0 core package. Astron. J. 156, 123 (2018).
Acknowledgements
This research was supported by World Premier International Research Center Initiative (WPI) (A.Y., S.T., T.O. and T.T.), MEXT, JAPAN, a matching fund programme of Centers for Inter-University Collaboration from ISAS/JAXA (A.Y, S.T. and T.O.) and MEXT JSPS KAKENHI grant numbers 18H02700 (S.T. and A.Y.), 18H05463 (T.T., A.Y., S.T., T.O. and M.K.) and 20K16692 (A.Y.).
Author information
Authors and Affiliations
Contributions
A.Y. initiated the project. A.Y. designed the experiments with help from H. Mizuma, Y.K. and H.F. A.Y., S.T., T.O., G.Y. and T.K. performed the experiments with help from I.O.U. and K.O., and H.F., S.T., M.K., G.Y. and H. Matsumura analysed the data. S.W., S.T., T.T. and A.Y. contributed materials/analysis tools. A.Y. wrote the paper with help from P.C., T.T., H.F., H. Mizuma and Y.K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Ramsey Badawi, Paul Lecoq and Marco Pizzichemi for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Source Data Fig. 2
Source data.
Rights and permissions
About this article
Cite this article
Yagishita, A., Takeda, S., Katsuragawa, M. et al. Simultaneous visualization of multiple radionuclides in vivo. Nat. Biomed. Eng 6, 640–647 (2022). https://doi.org/10.1038/s41551-022-00866-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00866-6
This article is cited by
-
Simultaneous quantitative imaging of two PET radiotracers via the detection of positron–electron annihilation and prompt gamma emissions
Nature Biomedical Engineering (2023)
-
Dual-radionuclide in vivo imaging of micro-metastasis and lymph tract with submillimetre resolution
Scientific Reports (2023)