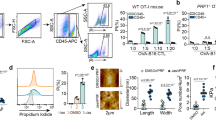
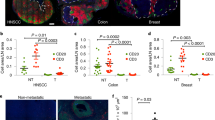
Abstract
Malignant transformation and tumour progression are associated with cancer-cell softening. Yet how the biomechanics of cancer cells affects T-cell-mediated cytotoxicity and thus the outcomes of adoptive T-cell immunotherapies is unknown. Here we show that T-cell-mediated cancer-cell killing is hampered for cortically soft cancer cells, which have plasma membranes enriched in cholesterol, and that cancer-cell stiffening via cholesterol depletion augments T-cell cytotoxicity and enhances the efficacy of adoptive T-cell therapy against solid tumours in mice. We also show that the enhanced cytotoxicity against stiffened cancer cells is mediated by augmented T-cell forces arising from an increased accumulation of filamentous actin at the immunological synapse, and that cancer-cell stiffening has negligible influence on: T-cell-receptor signalling, production of cytolytic proteins such as granzyme B, secretion of interferon gamma and tumour necrosis factor alpha, and Fas-receptor–Fas-ligand interactions. Our findings reveal a mechanical immune checkpoint that could be targeted therapeutically to improve the effectiveness of cancer immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data supporting the results in this study are available within the paper and its Supplementary Information. Source data for the figures are provided with this paper.
Code availability
Source code for the custom ImageJ macro for F-actin analysis, cellular-force calculation and deformability cytometry analysis, and custom MATLAB scripts for optical-tweezer data collection and data post-processing are available from the corresponding author on reasonable request. Shape-Out (version 2.7.4) for deformability cytometry analysis is available at https://github.com/ZELLMECHANIK-DRESDEN/ShapeOut2.
References
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Wang, H. & Mooney, D. J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 17, 761–772 (2018).
Eil, R. et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 537, 539–543 (2016).
André, P. et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 175, 1731–1743 (2018).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Cross, S. E., Jin, Y. S., Rao, J. & Gimzewski, J. K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2, 780–783 (2007).
Fritsch, A. et al. Are biomechanical changes necessary for tumour progression? Nat. Phys. 6, 730–732 (2010).
Swaminathan, V. et al. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 71, 5075–5080 (2011).
Alibert, C., Goud, B. & Manneville, J. B. Are cancer cells really softer than normal cells? Biol. Cell 109, 167–189 (2017).
Händel, C. et al. Cell membrane softening in human breast and cervical cancer cells. New J. Phys. 17, 083008 (2015).
Köster, D. V. & Mayor, S. Cortical actin and the plasma membrane: inextricably intertwined. Curr. Opin. Cell Biol. 38, 81–89 (2016).
Mossman, K. D., Campi, G., Groves, J. T. & Dustin, M. L. Altered TCR signaling from geometrically repatterned immunological synapses. Science 310, 1191–1193 (2005).
Judokusumo, E., Tabdanov, E., Kumari, S., Dustin, M. L. & Kam, L. C. Mechanosensing in T lymphocyte activation. Biophys. J. 102, L5–L7 (2012).
Liu, B., Chen, W., Evavold, B. D. & Zhu, C. Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell 157, 357–368 (2014).
Pan, Y. et al. Mechanogenetics for the remote and noninvasive control of cancer immunotherapy. Proc. Natl Acad. Sci. USA 115, 992–997 (2018).
Hickey, J. W. et al. Engineering an artificial T-cell stimulating matrix for immunotherapy. Adv. Mater. 31, 1807359 (2019).
Meng, K. P., Majedi, F. S., Thauland, T. J. & Butte, M. J. Mechanosensing through YAP controls T cell activation and metabolism. J. Exp. Med. 217, e20200053 (2020).
Basu, R. et al. Cytotoxic T cells use mechanical force to potentiate target cell killing. Cell 165, 100–110 (2016).
Hui, K. L., Balagopalan, L., Samelson, L. E. & Upadhyaya, A. Cytoskeletal forces during signaling activation in Jurkat T-cells. Mol. Biol. Cell 26, 685–695 (2014).
Saitakis, M. et al. Different TCR-induced T lymphocyte responses are potentiated by stiffness with variable sensitivity. Elife 6, e23190 (2017).
Byfield, F. J., Aranda-Espinoza, H., Romanenko, V. G., Rothblat, G. H. & Levitan, I. Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophys. J. 87, 3336–3343 (2004).
Khatibzadeh, N., Gupta, S., Farrell, B., Brownell, W. E. & Anvari, B. Effects of cholesterol on nano-mechanical properties of the living cell plasma membrane. Soft Matter 8, 8350–8360 (2012).
Behnke, O., Tranum-Jensen, J. & van Deurs, B. Filipin as a cholesterol probe. II. Filipin-cholesterol interaction in red blood cell membranes. Eur. J. Cell Biol. 35, 200–215 (1984).
Jambhekar, S. S. & Breen, P. Cyclodextrins in pharmaceutical formulations I: structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 21, 356–362 (2016).
Zidovetzki, R. & Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta Biomembr. 1768, 1311–1324 (2007).
Vahabikashi, A. et al. Probe sensitivity to cortical versus intracellular cytoskeletal network stiffness. Biophys. J. 116, 518–529 (2019).
Guo, M. et al. Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc. Natl Acad. Sci. USA 114, E8618–E8627 (2017).
Otto, O. et al. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat. Methods 12, 199–202 (2015).
Azadi, S., Tafazzoli-Shadpour, M., Soleimani, M. & Warkiani, M. E. Modulating cancer cell mechanics and actin cytoskeleton structure by chemical and mechanical stimulations. J. Biomed. Mater. Res. Part A 107A, 1569–1581 (2019).
Lekka, M. Discrimination between normal and cancerous cells using AFM. Bionanoscience 6, 65–80 (2016).
Butcher, D. T., Alliston, T. & Weaver, V. M. A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 (2009).
Zhu, M. et al. ACAT1 regulates the dynamics of free cholesterols in plasma membrane which leads to the APP-α-processing alteration. Acta Biochim. Biophys. Sin. 47, 951–959 (2015).
Yang, W. et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 531, 651–655 (2016).
Barry, M. & Bleackley, R. C. Cytotoxic T lymphocytes: all roads lead to death. Nat. Rev. Immunol. 2, 401–409 (2002).
Golstein, P. & Griffiths, G. M. An early history of T cell-mediated cytotoxicity. Nat. Rev. Immunol. 18, 527–535 (2018).
Chow, C. W., Rincón, M. & Davis, R. J. Requirement for transcription factor NFAT in interleukin-2 expression. Mol. Cell. Biol. 19, 2300–2307 (1999).
Oeckinghaus, A. & Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 1, a000034 (2009).
Style, R. W. et al. Traction force microscopy in physics and biology. Soft Matter 10, 4047–4055 (2014).
Weder, G. et al. Increased plasticity of the stiffness of melanoma cells correlates with their acquisition of metastatic properties. Nanomed. Nanotechnol. Biol. Med. 10, 141–148 (2014).
Luo, Q., Kuang, D., Zhang, B. & Song, G. Cell stiffness determined by atomic force microscopy and its correlation with cell motility. Biochim. Biophys. Acta Gen. Subj. 1860, 1953–1960 (2016).
Liu, Y. et al. Cell softness prevents cytolytic T-cell killing of tumor-repopulating cells. Cancer Res. 81, 476–488 (2021).
Blanchoin, L., Boujemaa-Paterski, R., Sykes, C. & Plastino, J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 94, 235–263 (2014).
Bashour, K. T. et al. CD28 and CD3 have complementary roles in T-cell traction forces. Proc. Natl Acad. Sci. USA 111, 2241–2246 (2014).
Vaahtomeri, K. et al. Locally triggered release of the chemokine CCL21 promotes dendritic cell transmigration across lymphatic endothelia. Cell Rep. 19, 902–909 (2017).
Leithner, A. et al. Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse. J. Cell Biol. 220, e202006081 (2021).
Stack, T. et al. Targeted delivery of cell softening micelles to Schlemm’s canal endothelial cells for treatment of glaucoma. Small 16, 2004205 (2020).
Vorselen, D. et al. Microparticle traction force microscopy reveals subcellular force exertion patterns in immune cell–target interactions. Nat. Commun. 11, 20 (2020).
Blumenthal, D., Chandra, V., Avery, L. & Burkhardt, J. K. Mouse T cell priming is enhanced by maturation-dependent stiffening of the dendritic cell cortex. Elife 9, e55995 (2020).
Tello-Lafoz, M. et al. Cytotoxic lymphocytes target characteristic biophysical vulnerabilities in cancer. Immunity 54, 1037–1054.e7 (2021).
Janmey, P. A. & McCulloch, C. A. Cell mechanics: integrating cell responses to mechanical stimuli. Annu. Rev. Biomed. Eng. 9, 1–34 (2007).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Huse, M. Mechanical forces in the immune system. Nat. Rev. Immunol. 17, 679–690 (2017).
Zhu, C., Chen, W., Lou, J., Rittase, W. & Li, K. Mechanosensing through immunoreceptors. Nat. Immunol. 20, 1269–1278 (2019).
Jain, N., Moeller, J. & Vogel, V. Mechanobiology of macrophages: how physical factors coregulate macrophage plasticity and phagocytosis. Annu. Rev. Biomed. Eng. 21, 267–297 (2019).
Lei, K., Kurum, A. & Tang, L. Mechanical immunoengineering of T cells for therapeutic applications. Acc. Chem. Res. 53, 2777–2790 (2020).
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 (2006).
Rosen, K., Shi, W., Calabretta, B. & Filmus, J. Cell detachment triggers p38 mitogen-activated protein kinase-dependent overexpression of Fas ligand: a novel mechanism of anoikis of intestinal epithelial cells. J. Biol. Chem. 277, 46123–46130 (2002).
Hawley, T. S. & Hawley, R. G. Flow Cytometry Protocols (Humana Press, 2018).
Tse, J. R. & Engler, A. J. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 47, 1–16 (2010).
Tseng, Q. et al. Spatial organization of the extracellular matrix regulates cell-cell junction positioning. Proc. Natl Acad. Sci.USA 109, 1506–1511 (2012).
Acknowledgements
We thank R. Guiet (EPFL) for assistance on image analysis; W. Li (West China Hospital) for histological analyses of human biopsies; the EPFL Bioimaging and Optics Platform, Center of PhenoGenomics, Flow Cytometry Core Facility, Protein Production and Structure Core Facility, and Histology Core Facility for technical support; S. M. Leitão and B. Ghadiani (EPFL) for technical assistance on AFM measurement; P. Müller (Max Planck Institute) for assistance on analysing deformability cytometry data; D. J. Irvine (MIT) for providing IL-15SA construct, B16F10-OVA, and 4T1-Fluc-tdTomato cell lines; P. Romero (UNIL) for providing MC38-HER2 and Me275-HER2 cell lines; D. Trono (EPFL) for providing HEK293T cells and pLKO.1 vector; E. Amstad (EPFL) for providing access to a rheometer; F. Stellacci, M. de Palma, A. Cont, and A. Persat (EPFL) for discussion. This work was supported in part by the European Research under the ERC grant agreements MechanoIMM (805337) and ROBOCHIP (714609), the Swiss National Science Foundation (Project grant 315230_173243), the Swiss Cancer Research Foundation (No. KFS-4600-08-2018), Kristian Gerhard Jebsen Foundation, Anna Fuller Fund Grant, and École Polytechnique Fédérale de Lausanne (EPFL). A.K. acknowledges funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 754354. M.G. was supported by the Chinese Scholarship Council (CSC) (No. 201808320453).
Author information
Authors and Affiliations
Contributions
K.L. and L.T. conceived the study and designed the experiments. K.L., A.K., M.K., L.B., Y.H., V.C., M.G., Y.-Q.X., Y.G., M.T.M.H., Y.W., G.Z., M.G., G.E.F., and M.S.S. performed the experiments. K.L., A.K., L.T., M.S.S., M.G., and G.E.F. analysed the data. L.T. supervised the project. K.L., A.K., and L.T. wrote the manuscript. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks Jochen Guck, Bo Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures.
Supplementary Data
Source data for the Supplementary figures.
Source data
Source Data Fig. 1
Source data.
Source Data Fig. 2
Source data.
Source Data Fig. 3
Source data.
Source Data Fig. 4
Source data.
Source Data Fig. 5
Source data.
Source Data Fig. 6
Source data.
Rights and permissions
About this article
Cite this article
Lei, K., Kurum, A., Kaynak, M. et al. Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy. Nat Biomed Eng 5, 1411–1425 (2021). https://doi.org/10.1038/s41551-021-00826-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00826-6
This article is cited by
-
PIEZO1 mechanically regulates the antitumour cytotoxicity of T lymphocytes
Nature Biomedical Engineering (2024)
-
Entry and exit of extracellular vesicles to and from the blood circulation
Nature Nanotechnology (2024)
-
Cell softness renders cytotoxic T lymphocytes and T leukemic cells resistant to perforin-mediated killing
Nature Communications (2024)
-
Targeting the mevalonate or Wnt pathways to overcome CAR T-cell resistance in TP53-mutant AML cells
EMBO Molecular Medicine (2024)
-
Evidence and therapeutic implications of biomechanically regulated immunosurveillance in cancer and other diseases
Nature Nanotechnology (2024)