Abstract
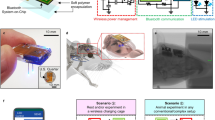
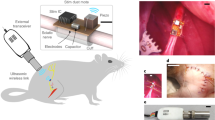
Implantable devices for the wireless modulation of neural tissue need to be designed for reliability, safety and reduced invasiveness. Here we report chronic electrical stimulation of the sciatic nerve in rats by an implanted organic electrolytic photocapacitor that transduces deep-red light into electrical signals. The photocapacitor relies on commercially available semiconducting non-toxic pigments and is integrated in a conformable 0.1-mm3 thin-film cuff. In freely moving rats, fixation of the cuff around the sciatic nerve, 10 mm below the surface of the skin, allowed stimulation (via 50–1,000-μs pulses of deep-red light at wavelengths of 638 nm or 660 nm) of the nerve for over 100 days. The robustness, biocompatibility, low volume and high-performance characteristics of organic electrolytic photocapacitors may facilitate the wireless chronic stimulation of peripheral nerves.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. All data generated in this study, including source data and the data used to make the figures, are available from figshare with the identifier https://doi.org/10.6084/m9.figshare.15015099. Source data are provided with this paper.
References
Krames, E. S., Peckham, P. H. & Rezai, A. R. (eds) Neuromodulation (Academic, 2009); https://doi.org/10.1023/B:MYCO.0000003704.30293.b6
Carrara, S. & Iniewski, K. (eds) Handbook of Bioelectronics (Cambridge Univ. Press, 2015); https://doi.org/10.1017/CBO9781139629539
Jastrzebska-Perfect, P. et al. Translational neuroelectronics. Adv. Funct. Mater. 30, 1909165 (2020).
Lozano, A. M. et al. Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol. 15, 148–160 (2019).
Chuang, A. T., Margo, C. E. & Greenberg, P. B. Retinal implants: a systematic review. Br. J. Ophthalmol. 98, 852–856 (2014).
Johnson, R. L. & Wilson, C. G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 11, 203–213 (2018).
Ben-Menachem, E., Revesz, D., Simon, B. J. & Silberstein, S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur. J. Neurol. 22, 1260–1268 (2015).
Caravaca, A. S. et al. An effective method for acute vagus nerve stimulation in experimental inflammation. Front. Neurosci. 13, 877 (2019).
Birmingham, K. et al. Bioelectronic medicines: a research roadmap. Nat. Rev. Drug Discov. 13, 399–400 (2014).
Acarón Ledesma, H. et al. An atlas of nano-enabled neural interfaces. Nat. Nanotechnol. 14, 645–657 (2019).
Tanabe, Y. et al. High-performance wireless powering for peripheral nerve neuromodulation systems. PLoS ONE 12, e0186698 (2017).
Larson, C. E. & Meng, E. A review for the peripheral nerve interface designer. J. Neurosci. Methods 332, 108523 (2020).
Thimot, J. & Shepard, K. L. Bioelectronic devices: wirelessly powered implants. Nat. Biomed. Eng. 1, 0051 (2017).
Agrawal, D. R. et al. Conformal phased surfaces for wireless powering of bioelectronic microdevices. Nat. Biomed. Eng. 1, 0043 (2017).
Lee, B. et al. An implantable peripheral nerve recording and stimulation system for experiments on freely moving animal subjects. Sci. Rep. 8, 6115 (2018).
Hernandez-Reynoso, A. G. et al. Miniature electroparticle-cuff for wireless peripheral neuromodulation. J. Neural Eng. 16, 046002 (2019).
Singer, A. B. et al. Magnetoelectric materials for miniature, wireless neural stimulation at therapeutic frequencies. Neuron 107, 631–643 (2020).
Cotero, V. et al. Noninvasive sub-organ ultrasound stimulation for targeted neuromodulation. Nat. Commun. 10, 952 (2019).
Piech, D. K. et al. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng. 4, 207–222 (2020).
Murakawa, K., Kobayashi, M., Nakamura, O. & Kawata, S. A wireless near-infrared energy system for medical implants. IEEE Eng. Med. Biol. 18, 70–72 (1999).
Kim, J. et al. Active photonic wireless power transfer into live tissues. Proc. Natl Acad. Sci. USA 117, 16856–16863 (2020).
Jacques, S. L. Optical properties of biological tissues: a review. Phys. Med. Biol. 58, 5007–5008 (2013).
Song, K. et al. Subdermal flexible solar cell arrays for powering medical electronic implants. Adv. Healthc. Mater. 5, 1572–1580 (2016).
Haeberlin, A. et al. The first batteryless, solar-powered cardiac pacemaker. Hear. Rhythm 12, 1317–1323 (2015).
Abdo, A. et al. Floating light-activated microelectrical stimulators tested in the rat spinal cord. J. Neural Eng. 8, 056012 (2011).
Yun, S. H. & Kwok, S. J. J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 1, 0008 (2017).
Park, S. et al. Self-powered ultra-flexible electronics via nano-grating-patterned organic photovoltaics. Nature 561, 516–521 (2018).
Jiang, Y. et al. Rational design of silicon structures for optically controlled multiscale biointerfaces. Nat. Biomed. Eng. 2, 508–521 (2018).
Scanziani, M. & Häusser, M. Electrophysiology in the age of light. Nature 461, 930–939 (2009).
Richter, C. P. & Tan, X. Photons and neurons. Hear. Res. 311, 72–88 (2014).
Shapiro, M. G., Homma, K., Villarreal, S., Richter, C.-P. & Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 3, 736 (2012).
Jiang, Y. et al. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces. Nat. Mater. 15, 1023–1030 (2016).
Jiang, Y. & Tian, B. Inorganic semiconductor biointerfaces. Nat. Rev. Mater. 3, 473–490 (2018).
Jiang, Y. et al. Nongenetic optical neuromodulation with silicon-based materials. Nat. Protoc. 14, 1339–1376 (2019).
Sytnyk, M. et al. Cellular interfaces with hydrogen-bonded organic semiconductor hierarchical nanocrystals. Nat. Commun. 8, 91 (2017).
Martino, N. et al. Photothermal cellular stimulation in functional bio-polymer interfaces. Sci. Rep. 5, 8911 (2015).
Wang, L. et al. Photovoltaic retinal prosthesis: implant fabrication and performance. J. Neural Eng. 9, 046014 (2012).
Ferlauto, L. et al. Design and validation of a foldable and photovoltaic wide-field epiretinal prosthesis. Nat. Commun. 9, 992 (2018).
Mathieson, K. et al. Photovoltaic retinal prosthesis with high pixel density. Nat. Photon. 6, 391–397 (2012).
Prévot, P. H. et al. Behavioural responses to a photovoltaic subretinal prosthesis implanted in non-human primates. Nat. Biomed. Eng. 4, 172–180 (2019).
Hopkins, J. et al. Photoactive organic substrates for cell stimulation: progress and perspectives. Adv. Mater. Technol. 4, 1800744 (2019).
Di Maria, F., Lodola, F., Zucchetti, E., Benfenati, F. & Lanzani, G. The evolution of artificial light actuators in living systems: from planar to nanostructured interfaces. Chem. Soc. Rev. 47, 4757–4780 (2018).
Sytnyk, M. et al. Hydrogen-bonded organic semiconductor micro- and nanocrystals: from colloidal syntheses to (opto-)electronic devices. J. Am. Chem. Soc. 136, 16522–16532 (2014).
Hunger, K. Toxicology and toxicological testing of colorants. Rev. Prog. Color. Relat. Top. 35, 76–89 (2005).
Rand, D. et al. Direct electrical neurostimulation with organic pigment photocapacitors. Adv. Mater. 30, 1707292 (2018).
Jakešová, M. et al. Optoelectronic control of single cells using organic photocapacitors. Sci. Adv. 5, eaav5265 (2019).
Ejneby, M. S. et al. Extracellular photovoltage clamp using conducting polymer-modified organic photocapacitors. Adv. Mater. Technol. 5, 1900860 (2020).
Merletti, R. & Parker, P. J. (eds) Electromyography Physiology, Engineering and Noninvasive Applications (Wiley, 2004).
Fortin, J. B. & Lu, T.-M. Chemical Vapor Polymerization, The Growth and Properties of Parylene (Springer, 2004).
Jacques, S. L. & Wang, L. MCML – Monte Carlo modeling of light transport in tissues. Comput. Methods Prog. Biomed. 47, 131–146 (1995).
Alerstam, E., Svensson, T. & Andersson-Engels, S. Parallel computing with graphics processing units for high-speed Monte Carlo simulation of photon migration. J. Biomed. Opt. 13, 060504 (2008).
Bashkatov, A. N., Genina, E. A. & Tuchin, V. V. Optical properties of skin, subcutaneous and muscle tissues: a review. J. Innov. Opt. Health Sci. 4, 9–38 (2011).
American National Standard for Safe Use of Lasers ANSI Z136 (Laser Institute of America, 2007).
Cogan, S. F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 10, 275–309 (2008).
Boehler, C., Carli, S., Fadiga, L., Stieglitz, T. & Asplund, M. Tutorial: guidelines for standardized performance tests for electrodes intended for neural interfaces and bioelectronics. Nat. Protoc. 15, 3557–3578 (2020).
Ðerek, V., Rand, D., Migliaccio, L., Hanein, Y. & Głowacki, E. D. Untangling photofaradaic and photocapacitive effects in organic optoelectronic stimulation devices. Front. Bioeng. Biotechnol. 8, 284 (2020).
Merrill, D. R., Bikson, M. & Jefferys, J. G. R. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J. Neurosci. Methods 141, 171–198 (2005).
Harnack, D. et al. The effects of electrode material, charge density and stimulation duration on the safety of high-frequency stimulation of the subthalamic nucleus in rats. J. Neurosci. Methods 138, 207–216 (2004).
Schoen, I. & Fromherz, P. Activation of Na+ channels in cell membrane by capacitive stimulation with silicon chip. Appl. Phys. Lett. 87, 193901–193903 (2005).
Seixas de Melo, J. et al. Photophysics of an indigo derivative (keto and leuco structures) with singular properties. J. Phys. Chem. A 110, 13653–13661 (2006).
Jeppesen, C., Mortensen, N. A. & Kristensen, A. The effect of Ti and ITO adhesion layers on gold split-ring resonators. Appl. Phys. Lett. 97, 2012–2015 (2010).
Benck, J. D., Pinaud, B. A., Gorlin, Y. & Jaramillo, T. F. Substrate selection for fundamental studies of electrocatalysts and photoelectrodes: inert potential windows in acidic, neutral and basic electrolyte. PLoS ONE 9, e107942 (2014).
Matarese, B. et al. Investigation of the stability and biocompatibility of commonly used electrode materials in organic neuro-optoelectronics. In Proc. 2015 IEEE 15th International Conference on Nanotechnology (IEEE-NANO) 1539–1542 (IEEE, 2015).
Selvakumaran, J., Hughes, M. P., Ewins, D. J. & Richards, P. R. Biocompatibility studies of materials used for chronically implantable microelectrodes. In Proc. 1st Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology 521–525 (IEEE, 2000); https://doi.org/10.1109/MMB.2000.893839
Ilic, B. & Craighead, H. G. Topographical patterning of chemically sensitive biological materials using a polymer-based dry lift off. Biomed. Microdev. 2, 317–322 (2000).
Yu, H., Xiong, W., Zhang, H., Wang, W. & Li, Z. A cable-tie-type parylene cuff electrode for peripheral nerve interfaces. In Proc. 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS) 9–12 (IEEE, 2014); https://doi.org/10.1109/MEMSYS.2014.6765560
Cobo, A. M. et al. Parylene-based cuff electrode with integrated microfluidics for peripheral nerve recording, stimulation and drug delivery. J. Microelectromech. Syst. 28, 36–49 (2019).
Günter, C., Delbeke, J. & Ortiz-Catalan, M. Safety of long-term electrical peripheral nerve stimulation: review of the state of the art. J. Neuroeng. Rehabil. 16, 13 (2019).
Ayaz, M. et al. Sexual dependency of rat sciatic nerve fiber conduction velocity distributions. Int. J. Neurosci. 117, 1537–1549 (2007).
Koo, Y. S., Cho, C. S. & Kim, B. J. Pitfalls in using electrophysiological studies to diagnose neuromuscular disorders. J. Clin. Neurol. 8, 1–14 (2012).
Metz, G. A. & Whishaw, I. Q. The ladder rung walking task: a scoring system and its practical application. J. Vis. Exp. 28, 1204 (2009).
Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M. & Yaksh, T. L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994).
Cameron, T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J. Neurosurg. 100, 254–267 (2004).
Perryman, L. T. Spinal cord stimulation costs and complications can be reduced by wireless nanotechnology. A review of traditional equipment expenses compared to wireless stimulation. Am. J. Anesth. Clin. Res. 4, 19–24 (2018).
Krook-Magnuson, E., Gelinas, J. N., Soltesz, I. & Buzsáki, G. Neuroelectronics and biooptics: closed-loop technologies in neurological disorders. JAMA Neurol. 72, 823–829 (2015).
Edwards, C. A., Kouzani, A., Lee, K. H. & Ross, E. K. Neurostimulation devices for the treatment of neurologic disorders. Mayo Clin. Proc. 92, 1427–1444 (2017).
Gutruf, P., Good, C. H. & Rogers, J. A. Perspective: implantable optical systems for neuroscience research in behaving animal models—current approaches and future directions. APL Photon. 3, 120901 (2018).
Lu, L. et al. Wireless optoelectronic photometers for monitoring neuronal dynamics in the deep brain. Proc. Natl Acad. Sci. USA 115, E1374–E1383 (2018).
Chang, J. H.-C., Lu, B. & Tai, Y.-C. Adhesion-enhancing surface treatments for parylene deposition. In Proc. 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference 390–393 (IEEE, 2011).
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 949191, E.D.G.). We acknowledge financial support from the Knut and Alice Wallenberg Foundation within the framework of the Wallenberg Centre for Molecular Medicine at Linköping University (E.D.G.), the Swedish Research Council (Vetenskapsrådet, 2018-04505, E.D.G.) and the Swedish Foundation for Strategic Research (E.D.G. and M.B.). This work was also supported by Columbia University, School of Engineering and Applied Science, as well as Columbia University Medical Center, Department of Neurology and Institute for Genomic Medicine. We acknowledge CzechNanoLab Research Infrastructure, supported by MEYS CR (LM2018110). This work has been supported by the Croatian Science Foundation under project UIP-2019-04-1753 (V.Đ.). V.Đ. acknowledges the support of project CeNIKS, co-financed by the Croatian Government and the European Union through the European Regional Development Fund – Competitiveness and Cohesion Operational Programme (grant no. KK.01.1.1.02.0013), and the QuantiXLie Center of Excellence, a project co-financed by the Croatian Government and European Union through the European Regional Development Fund – the Competitiveness and Cohesion Operational Programme (grant no. KK.01.1.1.01.0004). Financial support by the Center of Excellence for Advanced Materials and Sensors, Croatia, is gratefully acknowledged. We also thank H. Khodagholy for the design and fabrication of the horizontal ladder apparatus.
Author information
Authors and Affiliations
Contributions
M.S.E., M.J. and L.M. carried out the photoelectrochemical characterizations. M.S.E., M.J., V.Đ., D.K. and J.N.G. performed acute experiments and analysed data. L.M. fabricated the devices for the acute experiments. M.J. fabricated the devices for the chronic experiments. V.Đ. wrote programs for data acquisition, processing and MC modelling. I.S. performed calculations with finite-element models. Z.Z. designed and developed the electrophysiological acquisition hardware. J.J.F., D.K. and J.N.G. performed the rodent surgeries. Chronic data were collected and analysed by M.J., J.J.F., Z.Z., J.N.G. and E.D.G. J.J.F. and M.S.E. performed the rat behavioural testing. J.J.F. conducted immunohistochemistry experiments. The project was led and supervised by M.B., D.K., V.Đ., J.N.G. and E.D.G. The manuscript was written with input from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks Gregoire Courtine, John Ho and Bozhi Tian for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary appendix, figures, table and video captions.
Video 1
Initial acute photostimulation experiments, as described in Fig. 3.
Video 2
Leg-muscle twitches on the application of 1-ms light pulses at a frequency of 0.33 Hz, 14 days following implantation of the device.
Video 3
When the implant site is surgically opened and photostimulation is performed, clear muscle twitches are once again visible.
Video 4
Horizontal ladder test for rats with the implanted device versus control rats.
Source data
Source data for Fig. 2
Source data.
Source data for Fig. 3
Source data.
Source data for Fig. 4
Source data.
Source data for Fig. 5
Source data.
Rights and permissions
About this article
Cite this article
Silverå Ejneby, M., Jakešová, M., Ferrero, J.J. et al. Chronic electrical stimulation of peripheral nerves via deep-red light transduced by an implanted organic photocapacitor. Nat. Biomed. Eng 6, 741–753 (2022). https://doi.org/10.1038/s41551-021-00817-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00817-7
This article is cited by
-
Monolithic silicon for high spatiotemporal translational photostimulation
Nature (2024)
-
Switchable interfacial reaction enables bright and stable deep-red perovskite light-emitting diodes
Nature Photonics (2024)
-
A single n-type semiconducting polymer-based photo-electrochemical transistor
Nature Communications (2023)
-
Azobenzene-based optoelectronic transistors for neurohybrid building blocks
Nature Communications (2023)
-
Porosity-based heterojunctions enable leadless optoelectronic modulation of tissues
Nature Materials (2022)