Abstract
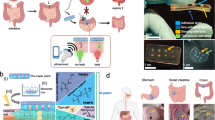
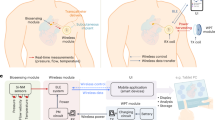
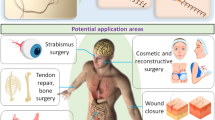
Monitoring surgical wounds post-operatively is necessary to prevent infection, dehiscence and other complications. However, the monitoring of deep surgical sites is typically limited to indirect observations or to costly radiological investigations that often fail to detect complications before they become severe. Bioelectronic sensors could provide accurate and continuous monitoring from within the body, but the form factors of existing devices are not amenable to integration with sensitive wound tissues and to wireless data transmission. Here we show that multifilament surgical sutures functionalized with a conductive polymer and incorporating pledgets with capacitive sensors operated via radiofrequency identification can be used to monitor physicochemical states of deep surgical sites. We show in live pigs that the sutures can monitor wound integrity, gastric leakage and tissue micromotions, and in rodents that the healing outcomes are equivalent to those of medical-grade sutures. Battery-free wirelessly operated bioelectronic sutures may facilitate post-surgical monitoring in a wide range of interventions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the paper and its supplementary information. Source data for Fig. 5f,g are provided with this paper. Other raw and analysed datasets generated during the study are available for research purposes from the corresponding authors on reasonable request.
References
Anderson, O., Davis, R., Hanna, G. B. & Vincent, C. A. Surgical adverse events: a systematic review. Am. J. Surg. 206, 253–262 (2013).
Maday, K. R., Hurt, J. B., Harrelson, P. & Porterfield, J. Evaluating postoperative fever. J. Am. Acad. Physician Assist. 29, 23–28 (2016).
Blouw, E. L., Rudolph, A. D., Narr, B. J. & Sarr, M. G. The frequency of respiratory failure in patients with morbid obesity undergoing gastric bypass. AANA J. 71, 45–50 (2003).
Endara, S. A., Serrano, A. J., Sandoval, B. A. & Davalos, G. A. Esophageal perforation during gastric bypass: delayed diagnosis and management. Obes. Surg. 17, 986–988 (2007).
Kazaure, H. S., Roman, S. A. & Sosa, J. A. Association of postdischarge complications with reoperation and mortality in general surgery. Arch. Surg. 147, 1000–1007 (2012).
Woodfield, J. C., Jamil, W. & Sagar, P. M. Incidence and significance of postoperative complications occurring between discharge and 30 days: a prospective cohort study. J. Surg. Res. 206, 77–82 (2016).
Pour-Ghaz, I., Hana, D., Raja, J., Ibebuogu, U. N. & Khouzam, R. N. CardioMEMS: where we are and where can we go? Ann. Transl. Med. 7, 418 (2019).
Steiger, C. et al. Ingestible electronics for diagnostics and therapy. Nat. Rev. Mater. 4, 83–98 (2019).
Kong, Y. L. et al. 3D-printed gastric resident electronics. Adv. Mater. Technol. 4, 1800490 (2019).
Someya, T., Bao, Z. & Malliaras, G. G. The rise of plastic bioelectronics. Nature 540, 379–385 (2016).
Yang, Y. & Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 48, 1465–1491 (2019).
Choi, S. et al. Highly conductive, stretchable and biocompatible Ag–Au core–sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 13, 1048–1056 (2018).
Chung, H. U. et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363, eaau0780 (2019).
Boutry, C. M. et al. A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat. Electron. 1, 314–321 (2018).
Tao, H. et al. Silk-based resorbable electronic devices for remotely controlled therapy and in vivo infection abatement. Proc. Natl Acad. Sci. USA 111, 17385–17389 (2014).
Wang, C. et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2, 687–695 (2018).
Boutry, C. M. et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 3, 47–57 (2019).
Burton, A. et al. Wireless, battery-free subdermally implantable photometry systems for chronic recording of neural dynamics. Proc. Natl Acad. Sci. USA 117, 2835–2845 (2020).
Kim, D.-H. et al. Thin, flexible sensors and actuators as ‘instrumented’ surgical sutures for targeted wound monitoring and therapy. Small 8, 3263–3268 (2012).
Mostafalu, P. et al. A toolkit of thread-based microfluidics, sensors, and electronics for 3D tissue embedding for medical diagnostics. Microsyst. Nanoeng. 2, 16039 (2016).
Wang, L. et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 4, 159–171 (2020).
Owyeung, R. E., Terse-Thakoor, T., Rezaei Nejad, H., Panzer, M. J. & Sonkusale, S. R. Highly flexible transistor threads for all-thread based integrated circuits and multiplexed diagnostics. ACS Appl. Mater. Interfaces 11, 31096–31104 (2019).
Terse-Thakoor, T. et al. Thread-based multiplexed sensor patch for real-time sweat monitoring. npj Flex. Electron. 4, 18 (2020).
Liu, M. et al. Biomimicking antibacterial opto-electro sensing sutures made of regenerated silk proteins. Adv. Mater. 33, 2004733 (2021).
Stuart, T., Cai, L., Burton, A. & Gutruf, P. Wireless, battery-free platforms for collection of biosignals. Biosens. Bioelectron. 178, 113007 (2021).
Mondal, S. & Chahal, P. A passive harmonic RFID tag and interrogator development. IEEE J. Radio Freq. Identif. 3, 98–107 (2019).
Vera, G. A., Duroc, Y. & Tedjini, S. Third harmonic exploitation in passive UHF RFID. IEEE Trans. Microw. Theory Tech. 63, 2991–3004 (2015).
Li, P., An, Z., Yang, L., Yang, P. & Lin, Q. RFID harmonic for vibration sensing. IEEE Trans. Mob. Comput. 20, 1614–1626 (2019).
Wang, Y. et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 3, e1602076 (2017).
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 17086 (2018).
Maehara, Y. et al. Impact of intra-abdominal absorbable sutures on surgical site infection in gastrointestinal and hepato-biliary-pancreatic surgery: results of a multicenter, randomized, prospective, phase II clinical trial. Surg. Today 47, 1060–1071 (2017).
Pirtea, L., Balint, O., Secosan, C., Grigoras, D. & Ilina, R. Laparoscopic pectopexy with burch colposuspension for pelvic prolapse associated with stress urinary incontinence. J. Minim. Invasive Gynecol. 27, 1023–1024 (2020).
IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz IEEE Std C95.1-2005 (IEEE, 2006).
Gabriel, S., Lau, R. W. & Gabriel, C. The dielectric properties of biological tissues: III. parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 41, 2271–2293 (1996).
Keat Ghee, O., Kefeng, Z. & Grimes, C. A. A wireless, passive carbon nanotube-based gas sensor. IEEE Sens. J. 2, 82–88 (2002).
Feng, Y., Xie, L., Chen, Q. & Zheng, L. Low-cost printed chipless RFID humidity sensor tag for intelligent packaging. IEEE Sens. J. 15, 3201–3208 (2015).
Mannoor, M. S., Zhang, S., Link, A. J. & McAlpine, M. C. Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc. Natl Acad. Sci. USA 107, 19207–19212 (2010).
Foltz, E. et al. An assessment of human gastric fluid composition as a function of PPI usage. Physiol. Rep. 3, e12269 (2015).
Engelking, L. R. Textbook of Veterinary Physiological Chemistry 3rd edn (Academic Press, 2015).
Sharma, P., Garg, N., Sharma, A., Capalash, N. & Singh, R. Nucleases of bacterial pathogens as virulence factors, therapeutic targets and diagnostic markers. Int. J. Med. Microbiol. 309, 151354 (2019).
Giacometti, A. et al. Epidemiology and microbiology of surgical wound infections. J. Clin. Microbiol. 38, 918–922 (2000).
Backes, F. J., Cohn, D. E., Mannel, R. S. & Fowler, J. M. in Clinical Gynecologic Oncology 9th edn (eds DiSaia, J. P. et al.) 560–578.e511 (Elsevier, 2018).
Bège, T. et al. An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest. Endosc. 73, 238–244 (2011).
Karmakar, N. C., Koswatta, R., Kalansuriya, P. & Rubayet, E. Chipless RFID Reader Architecture (Artech House, 2013).
Li, Y. et al. Very early colorectal anastomotic leakage within 5 post-operative days: a more severe subtype needs relaparatomy. Sci. Rep. 7, 39936 (2017).
Slieker, J. C., Daams, F., Mulder, I. M., Jeekel, J. & Lange, J. F. Systematic review of the technique of colorectal anastomosis. JAMA Surg. 148, 190–201 (2013).
Gonzalez, R. et al. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J. Am. Coll. Surg. 204, 47–55 (2007).
Han, S., Proctor, A. R., Ren, J., Benoit, D. S. W. & Choe, R. Temporal blood flow changes measured by diffuse correlation tomography predict murine femoral graft healing. PLoS ONE 13, e0197031 (2018).
Barbu, A., Jansson, L., Sandberg, M., Quach, M. & Palm, F. The use of hydrogen gas clearance for blood flow measurements in single endogenous and transplanted pancreatic islets. Microvasc. Res. 97, 124–129 (2015).
Wu, F. et al. Conductivity enhancement of PEDOT:PSS via addition of chloroplatinic acid and its mechanism. Adv. Electron. Mater. 3, 1700047 (2017).
Ho, J. S. et al. Wireless power transfer to deep-tissue microimplants. Proc. Natl Acad. Sci. USA 111, 7974–7979 (2014).
Agrawal, D. R. et al. Conformal phased surfaces for wireless powering of bioelectronic microdevices. Nat. Biomed. Eng. 1, 0043 (2017).
Lee, J. et al. Stretchable and suturable fibre sensors for wireless monitoring of connective tissue strain. Nat. Electron. 4, 291–301 (2021).
Cha, G. D., Kang, D., Lee, J. & Kim, D.-H. Bioresorbable electronic implants: history, materials, fabrication, devices, and clinical applications. Adv. Healthc. Mater. 8, 1801660 (2019).
Kang, S.-K. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016).
Hwang, S.-W. et al. A physically transient form of silicon electronics. Science 337, 1640–1644 (2012).
Dorsett-Martin, W. A. Rat models of skin wound healing: a review. Wound Repair Regen. 12, 591–599 (2004).
Obuobi, S. et al. Phenylboronic acid functionalized polycarbonate hydrogels for controlled release of polymyxin B in Pseudomonas aeruginosa infected burn wounds. Adv. Healthc. Mater. 7, 1701388 (2018).
Abramov, Y. et al. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen. 15, 80–86 (2007).
Topuz, F. & Okay, O. Rheological behavior of responsive DNA hydrogels. Macromolecules 41, 8847–8854 (2008).
Raymer, D. M. & Smith, D. E. Spontaneous knotting of an agitated string. Proc. Natl Acad. Sci. USA 104, 16432–16437 (2007).
Acknowledgements
We thank Z. Goh for assisting in the art in Fig. 1a; A. Bansal and H. Li for supporting the in vitro experiments; and Y. X. Guo for facilitating the dielectric measurements. J.S.H. acknowledges support from grants from the National Research Foundation Singapore (NRFF2017-07 and AISG-GC-2019-002), Ministry of Education Singapore (MOE2016-T3-1-004) and the Institute for Health Innovation and Technology. P.L.R.E. acknowledges funding provided by the Ministry of Education Singapore (R148000240114). Part of the work was performed at the National University of Singapore Medicine Confocal Microscopy Unit.
Author information
Authors and Affiliations
Contributions
V.K., X.Y., Z.X. and J.S.H. designed and performed the research. R.R.L., J.-W.W. and C.J.C. performed the large-animal studies. H.Y., H.G. and B.C.K.T. performed the mechanical testing experiments. R.R. conducted the histopathological studies. S.O., P.S., D.M., P.L.R.E. and W.L. performed the in vitro experiments and contributed materials. X.G. and J.O. assisted in the design of the fabrication process and contributed materials. X.T., S.A.K. and Z.L. supported the design and characterization of the wireless system. C.S.C. contributed to the study design. V.K., Z.X. and J.S.H. wrote the paper with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks Keat Ghee Ong, Sameer Sonkusale and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Radio-frequency response of different stitches and varying suture conductivity.
a-c, Current distribution on (a) Lembert, (b) lock, and (c) Cushing stitches at the fundamental f0 and harmonic 2f0 frequencies. The stitches are excited by a plane wave. d, Simulated received power detected by the wireless system at the second harmonic for Cushing stitch with varying conductivity at distance d from the antenna.
Extended Data Fig. 2 Effect of tissue curvature and spacing between adjacent sutures.
a,b, Schematic of the experimental setup for in-plane bending (a) and out-of-plane bending (b). c,d, Resonant frequency and averaged power over the operational band (2.2–3.4 GHz) measured for varying in-plane (c) and out-of-plane (d) bending angles. Error bars show the mean ± s.d. (n = 3 samples). e,f, Schematic diagrams of the test setup for wireless interference of WiSe with different spacing in X direction (e) and Y direction (f). WiSe sutures are separately labelled as WiSe1 and WiSe2. g,h, Measured harmonic backscattering spectra with various spacing in X direction (g) and Y direction (h).
Extended Data Fig. 3 Effect of suture length.
a,d Schematic of the experimental setup for suture with double-side (a) or single-side (d) cutting. The suture is placed under 2.5 cm porcine tissue and the length of varied Lembert stitches. b,e Averaged received power and received harmonic power (at 2.4 GHz) of WiSe over the operation band with double-side (b) or single-side (e) cutting. Error bars show the mean ± s.d. (n = 3 samples). c,f Harmonic signal received (at 2.4 GHz) for sutures with length 0, 10, and 20 mm on each side (c) or left side (f) of the pledget.
Extended Data Fig. 4 WiSe suture breakage test.
a, Schematic of test setup for simulating suture breakage under 2.5 cm porcine tissue. b, Heatmap of the received harmonic signal power as a function of the length of the left segment of the suture L' and the angle θ of the unravelled segment. c, Corresponding measured harmonic signal at 2.4 GHz. The signals are normalized to the initial suture state (0 dB). The angle θ is used to vary the effective length of the dipole antenna formed by the suture. In clinical applications, the unravelled segment is expected to spontaneously bunch together due to agitation by natural body motions61, which also leads to reduction of the effective dipole antenna length.
Extended Data Fig. 5 in vivo post-operative monitoring in rat model.
a, Illustration of the rodent surgical wound model. WiSe sutures were used to close an incision on the gluteal muscle and the skin over the wound stitched with unmodified silk sutures. Leakage of gastric fluid is simulated by subcutaneous injection of artificial gastric solution and breakage of the suture by cutting near the center of the surgical stitch. b, Computed tomography image of the surgical site. Dashed lines show WiSe suture estimated from the position of electronic pledget. c-e, Frequency-resolved wireless readout of the WiSe suture during implantation (c), gastric leakage (d), and suture breakage (e). Signal amplitudes were separately normalized based on the minimum amplitude of each group. f-h, Time-resolved wireless readout of the WiSe suture during implantation (f), gastric leakage (g), and suture breakage (h). Lower panels show respiratory waveforms aligned and normalized to the peak. i-k, Spectrogram (continuous wavelet transform) of the time-resolved signal. Red arrows indicate spectral peaks corresponding to the respiratory rate (RR, 0.28 Hz) and its second and third harmonics.
Extended Data Fig. 6 Reader antenna positioning.
a, Illustration of the steps to position the reader antenna. b, Contour plot of the received harmonic signal power when the position of the antenna is scanned within a 40 mm × 40 mm area. c, Measured backscattering signal for the antenna positions in (b). Yellow shading indicates the 10 mm × 10 mm area with highest signal amplitude. d, Resonant frequency and received power as a function of the orientation angle of the antenna. Blue shading denotes the frequency uncertainty due to decrease in the signal-to-noise ratio. e, Harmonic backscattering spectra for varying orientation angles.
Extended Data Fig. 7 Chronic wireless sensing in vivo.
a, Time-resolved wireless readout of WiSe suture applied to muscle wound on day 1, day 14, after simulated gastric leakage on day 14, and after simulated suture breakage on day 14. b, Signal-to-noise ratio (SNR) of wireless readout from WiSe sutures applied to skin wounds on rats over 14 days. Sutures are naturally removed by the rats as the skin wound heals. Box plots show the mean, upper quartile, and lower quartile (n = 5 rats on day 1 and n = 1 rat on day 14). c, Backscattering signals from a WiSe suture applied to a muscle wound over 14 days. Dash line indicates the harmonic signal amplitude on day 1. d,e, Representative H&E-stained tissue sections from the skin and muscle wounds near the sutures. Solid black arrows show skin re-epithelization (d), dashed black arrows show wound closure in muscle (e). Scale bars, 500 μm.
Supplementary information
Supplementary Information
Supplementary notes, figures, tables, references and video captions.
Supplementary Video 1
Suturing technique by threading the electronic pledget.
Supplementary Video 2
Suturing technique by knotting the electronic pledget.
Supplementary Video 3
Real-time wireless response of a surgical stitch.
Supplementary Video 4
Frequency-resolved wireless readout of a deep surgical stitch.
Supplementary Video 5
Ultrasound imaging of a suture in a porcine model.
Source data
Source Data for Fig. 5
Source data for Fig. 5f,g.
Rights and permissions
About this article
Cite this article
Kalidasan, V., Yang, X., Xiong, Z. et al. Wirelessly operated bioelectronic sutures for the monitoring of deep surgical wounds. Nat Biomed Eng 5, 1217–1227 (2021). https://doi.org/10.1038/s41551-021-00802-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00802-0
This article is cited by
-
Two way workable microchanneled hydrogel suture to diagnose, treat and monitor the infarcted heart
Nature Communications (2024)
-
Highly Elastic, Bioresorbable Polymeric Materials for Stretchable, Transient Electronic Systems
Nano-Micro Letters (2024)
-
A temperature-responsive intravenous needle that irreversibly softens on insertion
Nature Biomedical Engineering (2023)
-
Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing
Nature Biotechnology (2023)
-
Aligned nanofibrous collagen membranes from fish swim bladder as a tough and acid-resistant suture for pH-regulated stomach perforation and tendon rupture
Biomaterials Research (2022)