Abstract
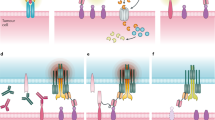
Bispecific T-cell engagers (BiTEs) preferentially targeting tumour-associated antigens and stimulating CD3-mediated signalling are being used in patients to treat acute B-cell lymphoblastic leukemia. However, the potency of BiTEs in solid tumours is limited by their short half-life and their severe toxicity at relevant therapeutic doses. Here we report the design and in vivo performance of a bispecific antibody that simultaneously targets the murine T-cell co-receptor CD3ε and the murine immune checkpoint programmed-death ligand 1 (PD-L1). In multiple syngeneic tumour models, the bispecific antibody generated higher antitumour immune responses than conventional BiTEs targeting tumour-associated antigens and CD3ε. We found that the durable antigen-specific T-cell responses resulted from the rejuvenation of CD8 T cells, owing to the blockade of PD-L1 on dendritic cells (but not on tumour cells) and co-stimulation by B7-1&2 (a peripheral membrane protein on dendritic cells). Bispecific T-cell engagers targeting dendritic cells rather than tumour cells may represent a general means of T-cell rejuvenation for durable cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw datasets generated during the study are too large to be publicly shared, but they are available for research purposes from the corresponding authors on reasonable request. Source data for the figures are available from figshare with the identifier https://doi.org/10.6084/m9.figshare.14984793.
References
Staerz, U. D., Kanagawa, O. & Bevan, M. J. Hybrid antibodies can target sites for attack by T cells. Nature 314, 628–631 (1985).
Garber, K. Bispecific antibodies rise again. Nat. Rev. Drug Discov. 13, 799–801 (2014).
Baeuerle, P. A. & Reinhardt, C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 69, 4941–4944 (2009).
Trabolsi, A., Arumov, A. & Schatz, J. H. T cell-activating bispecific antibodies in cancer therapy. J. Immunol. 203, 585–592 (2019).
Bargou, R. et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321, 974–977 (2008).
Topp, M. S. et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 16, 57–66 (2015).
Maude, S. L., Barrett, D., Teachey, D. T. & Grupp, S. A. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 20, 119–122 (2014).
Topp, M. S. et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J. Clin. Oncol. 32, 4134–4140 (2014).
Li, J. et al. CD3 bispecific antibody-induced cytokine release is dispensable for cytotoxic T cell activity. Sci. Transl. Med. 11, eaax8861 (2019).
Reusch, U. et al. Anti-CD3 × anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin. Cancer Res. 12, 183–190 (2006).
Cioffi, M., Dorado, J., Baeuerle, P. A. & Heeschen, C. EpCAM/CD3-bispecific T-cell engaging antibody MT110 eliminates primary human pancreatic cancer stem cells. Clin. Cancer Res. 18, 465–474 (2012).
Han, H. et al. Bispecific anti-CD3 × anti-HER2 antibody mediates T cell cytolytic activity to HER2-positive colorectal cancer in vitro and in vivo. Int. J. Oncol. 45, 2446–2454 (2014).
Kebenko, M. et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE(R)) antibody construct, in patients with refractory solid tumors. Oncoimmunology 7, e1450710 (2018).
Lutterbuese, R. et al. T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proc. Natl Acad. Sci. USA 107, 12605–12610 (2010).
van Panhuys, N. TCR signal strength alters T-DC activation and interaction times and directs the outcome of differentiation. Front. Immunol. 7, 6 (2016).
Chai, J. G. & Lechler, R. I. Immobilized anti-CD3 mAb induces anergy in murine naive and memory CD4+ T cells in vitro. Int. Immunol. 9, 935–944 (1997).
Harding, F. A., McArthur, J. G., Gross, J. A., Raulet, D. H. & Allison, J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 356, 607–609 (1992).
Green, D. R., Droin, N. & Pinkoski, M. Activation-induced cell death in T cells. Immunol. Rev. 193, 70–81 (2003).
Curtsinger, J. M. & Mescher, M. F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 22, 333–340 (2010).
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 (2011).
MacKay, M. et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nat. Biotechnol. 38, 233–244 (2020).
Labrijn, A. F., Janmaat, M. L., Reichert, J. M. & Parren, P. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug Discov. 18, 585–608 (2019).
Seckinger, A. et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell 31, 396–410 (2017).
Rius Ruiz, I. et al. p95HER2-T cell bispecific antibody for breast cancer treatment. Sci. Transl. Med. 10, eaat1445 (2018).
Boise, L. H. et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity 3, 87–98 (1995).
Garfall, A. L. & June, C. H. Trispecific antibodies offer a third way forward for anticancer immunotherapy. Nature 575, 450–451 (2019).
Skokos, D. et al. A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Sci. Transl. Med. 12, eaaw7888 (2020).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Wu, L. et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat. Cancer 1, 86–98 (2020).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Knutson, K. L. & Disis, M. L. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 54, 721–728 (2005).
Jansen, C. S. et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576, 465–470 (2019).
Zou, W., Wolchok, J. D. & Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 8, 328rv324 (2016).
Lin, H. et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J. Clin. Investig. 128, 805–815 (2018).
Tang, H. et al. PD-L1 on host cells is essential for PD-L1 blockade–mediated tumor regression. J. Clin. Investig. 128, 580–588 (2018).
Garcia-Diaz, A. et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 19, 1189–1201 (2017).
Kohnke, T., Krupka, C., Tischer, J., Knosel, T. & Subklewe, M. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J. Hematol. Oncol. 8, 111 (2015).
Kobold, S., Pantelyushin, S., Rataj, F. & Vom Berg, J. Rationale for combining bispecific T cell activating antibodies with checkpoint blockade for cancer therapy. Front. Oncol. 8, 285 (2018).
Schlothauer, T. et al. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Eng. Des. Sel. 29, 457–466 (2016).
Wei, H. et al. Structural basis of a novel heterodimeric Fc for bispecific antibody production. Oncotarget 8, 51037–51049 (2017).
Qiao, J. et al. Targeting tumors with IL-10 prevents dendritic cell-mediated CD8+ T cell apoptosis. Cancer Cell 35, 901–915.e4 (2019).
Benonisson, H. et al. CD3-bispecific antibody therapy turns solid tumors into inflammatory sites but does not install protective memory. Mol. Cancer Ther. 18, 312–322 (2019).
Sade-Feldman, M. et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175, 998–1013.e20 (2018).
Diskin, B. et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat. Immunol. 21, 442–454 (2020).
Hui, E. et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355, 1428–1433 (2017).
Kamphorst, A. O. et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 355, 1423–1427 (2017).
Kelly, E., Won, A., Refaeli, Y. & van Parijs, L. IL-2 and related cytokines can promote T cell survival by activating AKT. J. Immunol. 168, 597–603 (2002).
Heiss, M. M. et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int. J. Cancer 127, 2209–2221 (2010).
Kiewe, P. et al. Phase I trial of the trifunctional anti-HER2 × anti-CD3 antibody ertumaxomab in metastatic breast cancer. Clin. Cancer Res. 12, 3085–3091 (2006).
Schildberg, F. A., Klein, S. R., Freeman, G. J. & Sharpe, A. H. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity 44, 955–972 (2016).
Horn, L. A. et al. CD3×PDL1 bi-specific T cell engager (BiTE) simultaneously activates T cells and NKT cells, kills PDL1+ tumor cells, and extends the survival of tumor-bearing humanized mice. Oncotarget 8, 57964–57980 (2017).
Mayoux, M. et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci. Transl. Med. 12, eaav7431 (2020).
Zhao, Y. et al. PD-L1:CD80 cis-heterodimer triggers the co-stimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity 51, 1059–1073.e9 (2019).
Rafiq, S. et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 36, 847–856 (2018).
Hill, B. T., Roberts, Z. J., Xue, A., Rossi, J. M. & Smith, M. R. Rapid tumor regression from PD-1 inhibition after anti-CD19 chimeric antigen receptor T-cell therapy in refractory diffuse large B-cell lymphoma. Bone Marrow Transplant. 55, 1184–1187 (2019).
Wallberg, M. et al. Anti-CD3 treatment up-regulates programmed cell death protein-1 expression on activated effector T cells and severely impairs their inflammatory capacity. Immunology 151, 248–260 (2017).
del Rio, M. L., Bernhardt, G., Rodriguez-Barbosa, J. I. & Forster, R. Development and functional specialization of CD103+ dendritic cells. Immunol. Rev. 234, 268–281 (2010).
Refaeli, Y., van Parijs, L., Alexander, S. I. & Abbas, A. K. Interferon γ is required for activation-induced death of T lymphocytes. J. Exp. Med. 196, 999–1005 (2002).
Liu, X. et al. Dual targeting of innate and adaptive checkpoints on tumor cells limits immune evasion. Cell Rep. 24, 2101–2111 (2018).
Acknowledgements
We thank the UT Southwestern Institutional Animal Care & Use Committee (IACUC) and the Animal Resources Center (ARC). Y.-X.F. holds the Mary Nell and Ralph B. Rogers Professorship in Immunology. This work was supported by the Cancer Prevention and Research Institute of Texas (CPRIT) grant RR150072, given to Y.-X.F., and the National Institutes of Health (NIH) grant 1U54CA244719-01, to J.Q. We also thank Y. Liang, X. Cao, Z. Ren, A. Zhang, C. Dong, Z. Liu, C. Lu and B. Moon for providing experiment materials and helpful discussions.
Author information
Authors and Affiliations
Contributions
L.L. and Y.-X.F. designed the study and analysed the data. L.L. performed the experiments. J.C. and J.B. assisted with tumour experiments. H.L. assisted with tissue staining. Z.S. assisted with bispecific antibody purification. C.H. provided mice and reagents. L.L. and Y.-X.F. wrote the manuscript. C.M., E.H. and J.Q. revised the manuscript. J.Q. assisted with data interpretation. Y.-X.F. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks Marion Subklewe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
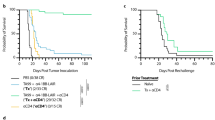
Extended Data Fig. 1 PD-L1xCD3 generates superior antitumour effects than TAA-targeting BiTE in vivo.
a-b, C57BL/6 J mice were subcutaneously inoculated with 3 × 105 MC38E5 tumor cells and treated with 0.25 mg kg−1 of bispecific antibodies twice on day 10 and 15. Tumor volume was measured twice per week (a). 60 days post treatment, tumor free mice were re-challenged with 3 × 106 tumor cells (b). c-d, C57BL/6 J mice were subcutaneously inoculated with 1 × 106 TC1E5 tumor cells and treated with 0.25 mg kg−1 of bispecific antibodies twice on day 10 and 15. Tumor volume was measured twice per week (c). 60 days post treatment, tumor free mice were re-challenged with 1 × 107 tumor cells (d). e, C57BL/6 J mice were subcutaneously inoculated with 3 × 105 B16E5 tumor cells and treated with 0.25 mg kg−1 of bispecific antibodies intraperitoneally twice on day 8 and 12. f, BALB/c mice were subcutaneously inoculated with 5 × 105 TuBoE5 tumor cells and treated with 0.25 mg kg−1 of fusion proteins intraperitoneally twice on day 10 and 14. g, C57BL/6 J mice were subcutaneously inoculated with 3 × 105 MC38E5 tumor cells and treated with 0.25 mg kg−1 of fusion proteins either intratumorally or intraperitoneally twice on day 9 and 13. Data were presented as mean ± s.e.m from a representative experiment (n = 5 (a, b, f, g), 4 (c-e) biologically independent animals) of two independent experiments. Statistical analysis was performed by two-way ANOVA with Tukey’s multiple comparisons test. ****P ≤ 0.0001.
Extended Data Fig. 2 PD-L1xCD3 targets pre-existing CD8 T cells in the tumour tissue to initiate the antitumour immune response.
a-d, C57BL/6 J mice were inoculated with 1 × 106 MC38 tumor cells and treated with PD-L1xCD3 (0.25 mg kg−1 on day 10 and 15). 200 μg of anti-CD8, anti-CD4, anti-NK1.1 or 500 μg of anti-CSF1R was administrated respectively one day before treatment initiation and then twice a week for 2 weeks. The percentage of CD8 + cells (a), CD4 + cells (b), NK1.1+ cells (c) and CD11b + F4/80+ cells (d) in the spleen were detected by flow cytometry. e, C57BL/6 J mice (n = 5 biologically independent animals) were subcutaneously inoculated with 1 × 106 MC38 tumor cells and treated with 0.25 mg kg−1 of PD-L1xCD3 either intratumorally or intraperitoneally twice on day 10 and 15. f, C57BL/6 J mice (n = 3 biological replicates) were subcutaneously inoculated with 1 × 106 MC38 tumor cells and intraperitoneally treated with 0.25 mg kg−1 of PD-L1xCD3. Concentration of fusion protein in different tissues were measured by hIgG ELISA at indicated time point. Data were shown as mean ± s.e.m from a representative experiment of two independent experiments. Statistical analysis was performed by two-way ANOVA with Tukey’s multiple comparisons test. ****P ≤ 0.0001.
Extended Data Fig. 3 Co-stimulatory signaling blockade abolished PD-L1xCD3 mediated antitumour effects and the in vitro activation of T cells by TAA-targeting BiTE.
a-b, C57BL/6 J mice were inoculated with 1 × 106 MC38 tumor cells and treated with PD-L1xCD3 (0.25 mg kg−1 on day 10 and 15), 200 μg CTLA4-Ig was administrated on day 10, 13 and 15. Experimental design (a) and tumor growth curve (b) are shown. c-e, CD8 T cells were co-cultured with either tumor cells or dendritic cells in the presence of ErbxCD3. T cell activation (c), apoptotic T cells (d), supernatant IL-2 and IFN-γ (e) were measured by flow cytometry. Data were shown as mean ± s.e.m from a representative experiment of two independent experiments (n = 5 biologically independent animals). Statistical analyses were performed by two-way ANOVA with Dunnett’s multiple comparisons test (b), two-tailed unpaired Student’s t-test (c-e). ***P ≤ 0.001, ****P ≤ 0.0001.
Extended Data Fig. 4 Correlation analysis of CD28 expression with CD80/86 expression, dendritic cell infiltration and patient survival.
TCGA database was analyzed for cumulative survival according to CD28 expression (a), correlation of CD28 level with CD80 and CD86 level (b) and correlation of CD28 level with dendritic cell infiltration (c). Skin cutaneous melanoma (SKCM), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), lung adenocarcinoma (LUAD), colon adenocarcinoma (COAD), breast invasive carcinoma (BRCA). Statistical analyses were performed by log-rank test (a), and Spearman’s rho correlation test (b-c).
Supplementary information
Supplementary Information
Supplementary figures and tables.
Rights and permissions
About this article
Cite this article
Liu, L., Chen, J., Bae, J. et al. Rejuvenation of tumour-specific T cells through bispecific antibodies targeting PD-L1 on dendritic cells. Nat Biomed Eng 5, 1261–1273 (2021). https://doi.org/10.1038/s41551-021-00800-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00800-2
This article is cited by
-
Dendritic cells as orchestrators of anticancer immunity and immunotherapy
Nature Reviews Clinical Oncology (2024)
-
Nanobody-based trispecific T cell engager (Nb-TriTE) enhances therapeutic efficacy by overcoming tumor-mediated immunosuppression
Journal of Hematology & Oncology (2023)
-
IL-2 delivery by engineered mesenchymal stem cells re-invigorates CD8+ T cells to overcome immunotherapy resistance in cancer
Nature Cell Biology (2022)
-
Concurrent delivery of immune checkpoint blockade modulates T cell dynamics to enhance neoantigen vaccine-generated antitumor immunity
Nature Cancer (2022)
-
Multiplexed imaging mass cytometry reveals distinct tumor-immune microenvironments linked to immunotherapy responses in melanoma
Communications Medicine (2022)