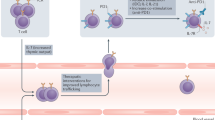
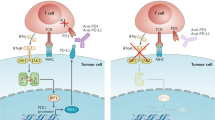
Abstract
Antigen release resulting from the death of tumour cells induced by chemotherapies and targeted therapies can augment the antitumour responses induced by immune checkpoint blockade (ICB). However, tumours responding to ICB therapies often become resistant to them. Here we show that the specific targeting of tumour cells promotes the growth of tumour-cell variants that are resistant to ICB, and that the acquired resistance can be overcome via the concurrent depletion of tumour cells and of major types of immunosuppressive cell via a monoclonal antibody binding the enzyme CD73, which we identified as highly expressed on tumour cells and on regulatory T cells, myeloid-derived suppressor cells and tumour-associated macrophages, but not on cytolytic T lymphocytes, natural killer cells and dendritic cells. In mice with murine tumours, the systemic administration of anti-PD1 antibodies and anti-CD73 antibodies conjugated to a near-infrared dye prevented near-infrared-irradiated tumours from acquiring resistance to ICB and resulted in the eradication of advanced tumours. The elimination of immunosuppressive cells may overcome acquired resistance to ICB across a range of tumour types and combination therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, but they are available for research purposes from the corresponding author on reasonable request. Source data are provided with this paper.
References
Reck, M. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Adams, S. et al. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-086 cohort A. J. Clin. Oncol. 35, 1008 (2017).
Vonderheide, R. H. et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin. Cancer Res. 16, 3485–3494 (2010).
Patnaik, A. et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin. Cancer Res. 21, 4286–4293 (2015).
Brahmer, J. R. et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Segal, N. et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J. Clin. Oncol. 32, 3002 (2014) .
Royal, R. E. et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 33, 828–833 (2010).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Granier, C. et al. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2, e000213 (2017).
Bjoern, J. et al. Immunological correlates of treatment and response in stage IV malignant melanoma patients treated with Ipilimumab. Oncoimmunology 5, e1100788 (2016).
Holmgaard, R. B., Zamarin, D., Lesokhin, A., Merghoub, T. & Wolchok, J. D. Targeting myeloid-derived suppressor cells with colony stimulating factor-1 receptor blockade can reverse immune resistance to immunotherapy in indoleamine 2,3-dioxygenase-expressing tumors. EBioMedicine 6, 50–58 (2016).
Meyer, C. et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol. Immunother. 63, 247–257 (2014).
Highfill, S. L. et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 6, 237ra67 (2014).
Gebhardt, C. et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin. Cancer Res. 21, 5453–5459 (2015).
Diaz-Montero, C. M., Finke, J. & Montero, A. J. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin. Oncol. 41, 174–184 (2014).
Kumar, V., Patel, S., Tcyganov, E. & Gabrilovich, D. I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 37, 208–220 (2016).
Talmadge, J. E. & Gabrilovich, D. I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 13, 739–752 (2013).
Colombo, M. P. & Piconese, S. Regulatory T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat. Rev. Cancer 7, 880–887 (2007).
Nishikawa, H. & Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Curr. Opin. Immunol. 27, 1–7 (2014).
Buckner, J. H. Mechanisms of impaired regulation by CD4+CD25+FOXP3+ regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol. 10, 849–859 (2010).
Sato, K., Nagaya, T., Mitsunaga, M., Choyke, P. L. & Kobayashi, H. Near infrared photoimmunotherapy for lung metastases. Cancer Lett. 365, 112–121 (2015).
Mitsunaga, M. et al. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 17, 1685–1691 (2011).
Sato, K. et al. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci. Transl. Med. 8, 352ra110 (2016).
Mellman, I., Coukos, G. & Dranoff, G. Cancer immunotherapy comes of age. Nature https://doi.org/10.1038/nature10673 (2011).
Li, P. et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature https://doi.org/10.1038/nature11530 (2012).
Elpek, K. G. et al. The tumor microenvironment shapes lineage, transcriptional, and functional diversity of infiltrating myeloid cells. Cancer Immunol. Res. https://doi.org/10.1158/2326-6066.CIR-13-0209 (2014).
Tuit, S. et al. Transcriptional signature derived from murine tumor-associated macrophages correlates with poor outcome in breast cancer patients. Cell Rep. https://doi.org/10.1016/j.celrep.2019.09.067 (2019).
Azambuja, J. H. et al. Blockade of CD73 delays glioblastoma growth by modulating the immune environment. Cancer Immunol. Immunother. https://doi.org/10.1007/s00262-020-02569-w (2020).
Goswami, S. et al. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat. Med. https://doi.org/10.1038/s41591-019-0694-x (2020).
Beavis, P. A., Stagg, J., Darcy, P. K. & Smyth, M. J. CD73: A potent suppressor of antitumor immune responses. Trends Immunol. 33, 231–237 (2012).
Buisseret, L. et al. Clinical significance of CD73 in triple-negative breast cancer: multiplex analysis of a phase III clinical trial. Ann. Oncol. https://doi.org/10.1093/annonc/mdx730 (2018).
Azad, A. et al. PD‐L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol. Med. 9, 167–180 (2017).
Corbett, T. H. et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 44, 717–726 (1984).
Allard, B., Pommey, S., Smyth, M. J. & Stagg, J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-13-0545 (2013).
Stagg, J. et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.0908801107 (2010).
de Mingo Pulido, Á. et al. TIM-3 regulates CD103+ dendritic cell function and response to chemotherapy in breast cancer. Cancer Cell https://doi.org/10.1016/j.ccell.2017.11.019 (2018).
Lin, E. Y. et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 163, 2113–2126 (2003).
Guy, C. T., Cardiff, R. D. & Muller, W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell Biol. 12, 954–961 (1992).
Uhlen, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Bedard, P. L., Hansen, A. R., Ratain, M. J. & Siu, L. L. Tumour heterogeneity in the clinic. Nature 501, 355–364 (2013).
Agarwal, R. & Kaye, S. B. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 3, 502–516 (2003).
Lackner, M. R., Wilson, T. R. & Settleman, J. Mechanisms of acquired resistance to targeted cancer therapies. Future Oncol. 8, 999–1014 (2012).
Neel, D. S. & Bivona, T. G. Resistance is futile: overcoming resistance to targeted therapies in lung adenocarcinoma. NPJ Precis. Oncol. 1, 3 (2017).
Vijayan, D., Young, A., Teng, M. W. L. & Smyth, M. J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 17, 709–724 (2017).
Chen, S. et al. CD73 expression on effector T cells sustained by TGF-β facilitates tumor resistance to anti-4-1BB/CD137 therapy. Nat. Commun. https://doi.org/10.1038/s41467-018-08123-8 (2019).
de Leve, S., Wirsdörfer, F. & Jendrossek, V. Targeting the immunomodulatory CD73/adenosine system to improve the therapeutic gain of radiotherapy. Front. Immunol. https://doi.org/10.3389/fimmu.2019.00698 (2019).
Zhang, B. CD73: a novel target for cancer immunotherapy. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-10-1544 (2010).
Leone, R. D. & Emens, L. A. Targeting adenosine for cancer immunotherapy. J. Immunother. Cancer https://doi.org/10.1186/s40425-018-0360-8 (2018).
Mixed reviews for A2AR inhibitor in NSCLC. Cancer Discov. 9, OF2 (2019).
Narravula, S., Lennon, P. F., Mueller, B. U. & Colgan, S. P. Regulation of endothelial CD73 by adenosine: paracrine pathway for enhanced endothelial barrier function. J. Immunol. https://doi.org/10.4049/jimmunol.165.9.5262 (2000).
Yu, M. et al. CD73 on cancer-associated fibroblasts enhanced by the A2B-mediated feedforward circuit enforces an immune checkpoint. Nat. Commun. https://doi.org/10.1038/s41467-019-14060-x (2020).
Seaman, S. et al. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell 31, 501–515.e8 (2017).
Keen, J. C. & Moore, H. M. The genotype-tissue expression (GTEx) project: linking clinical data with molecular analysis to advance personalized medicine. J. Pers. Med. 5, 22–29 (2015).
Yu, N. Y. L. et al. Complementing tissue characterization by integrating transcriptome profiling from the human protein atlas and from the FANTOM5 consortium. Nucleic Acids Res. 43, 6787–6798 (2015).
Sciarra, A. et al. CD73 expression in normal and pathological human hepatobiliopancreatic tissues. Cancer Immunol. Immunother. https://doi.org/10.1007/s00262-018-2290-1 (2019).
Bown, S. G. et al. Photodynamic therapy for cancer of the pancreas. Gut 50, 549–557 (2002).
Xue, G., Jin, G., Fang, J. & Lu, Y. IL-4 together with IL-1β induces antitumor Th9 cell differentiation in the absence of TGF-β signaling. Nat. Commun. 10, 1376 (2019).
Brinke, A. T. et al. Monitoring T-cell responses in translational studies: optimization of dye-based proliferation assay for evaluation of antigen-specific responses. Front. Immunol. https://doi.org/10.3389/fimmu.2017.01870 (2017).
Mao, C., Li, F., Zhao, Y., Debinski, W. & Ming, X. P-glycoprotein-targeted photodynamic therapy boosts cancer nanomedicine by priming tumor microenvironment. Theranostics https://doi.org/10.7150/thno.29580 (2018).
Jenkins, R. W. et al. Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov. 8, 196–215 (2018).
Ootani, A. et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. https://doi.org/10.1038/nm.1951 (2009).
Lu, Y. et al. Th9 cells promote antitumor immune responses in vivo. J. Clin. Invest. 122, 4160–4171 (2012).
Lu, Y. et al. Th9 cells represent a unique subset of CD4+ T cells endowed with the ability to eradicate advanced tumors. Cancer Cell 33, 1048–1060.e7 (2018).
Acknowledgements
This work was supported by grants from the National Cancer Institute (NCI, 4R00CA190910-03; 1R37CA251318-01; 1R01CA248111-01A1; R01 CA258477-01; 1R01CA264102-01), NCI P30 Administrative Supplement for Cell-Based Therapy (3P30CA012197-44S5), Daryl and Marguerite Errett Discovery Award 2020, ACS Research Scholar Grant (RSG-19-149-01-LIB), Wake Forest Baptist Comprehensive Cancer Center (WFBCCC) Push Pilot projects and CPRIT Scholar (RR210067). Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001420 (CTSI Pilot Grant Award 2018, CTSI Pilot Grant Award 2019 and CTSI Ignition Fund Pilot award). We thank I. M. Newman from the Wake Forest CTSI for manuscript editing assistance. This study was also supported by the NCI’s Cancer Center Support Grant award number P30CA012197 issued to the Wake Forest Baptist Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI.
Author information
Authors and Affiliations
Contributions
G.X. and Y.L. designed the experiments, analysed the data and wrote the paper. G.X. performed most of the experiments. G.J. helped with CyTOF data analysis. X.L. performed statistical analysis. J.F., Z.W. and N.Z. helped with animal studies. C.M. and X.M. provided technical support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 αCD73-Dye + NIR treatment shrinks advanced tumors in vivo.
(a) Treatment procedures of αCD73-Dye-mediated NIR irradiation against 4T1.2 and Pan02 tumors in vivo. NIR was given on tumors only, while other parts of the mice were shielded from light. Tumor growth curves of 4T1.2 (b) and Pan02 (c) tumors treated with IgG, IgG-Dye + NIR, αCD73-Dye, or αCD73-Dye + NIR (n=5 mice/group). The red arrow represents near-infrared (NIR) irradiation. Representative results from one of two repeated experiments are shown.
Extended Data Fig. 2 Changes in ratios of different immune cells in tumors after αCD73-Dye + NIR treatment.
CD8 + T cells to Treg cells ratio (a), CD8 + T cells to TAM.M2 cells ratio (b), CD8 + T cells to PMN.MDSC cells ratio (c), CD8 + T cells to Mo.MDSC cells ratio (d), NK cells to Treg cells ratio (e), NK cells to TAM.M2 cells ratio (f), NK cells to PMN.MDSC cells ratio (g), NK cells to Mo.MDSC cells ratio (h), and Foxp3.neg CD4 + T cells to Treg cells ratio (i) in 4T1.2 tumors after indicated treatments (as described in Extended Data Fig. 1) were tested by CyTOF (n = 3 biological replicates). The t-SNE plot of tumour-infiltrating CD45 + compartment overlaid with the expression of GzmB (j) and IFN-γ (k) from the 4T1.2 tumour after treatment. Data are mean ± SD. ****P < 0.0001, αCD73-Dye + NIR group compared with IgG, IgG-Dye + NIR, or αCD73-Dye group, one-way ANOVA with Tukey’s correction (a, b, c, d, and h). # means **P = 0.005834, αCD73-Dye + NIR group compared with IgG group, ## means **P = 0.006561, αCD73-Dye + NIR group compared with IgG-Dye + NIR group, ### means *P = 0.010734, αCD73-Dye + NIR group compared with αCD73-Dye group, one-way ANOVA with Tukey’s correction (f). # means **P = 0.002767, αCD73-Dye + NIR group compared with IgG group, ## means **P = 0.005519, αCD73-Dye + NIR group compared with IgG-Dye + NIR group, ### means *P = 0.028222, αCD73-Dye + NIR group compared with αCD73-Dye group, one-way ANOVA with Tukey’s correction (g). # means **P = 0.007576, αCD73-Dye + NIR group compared with IgG group, ## means **P = 0.004057, αCD73-Dye + NIR group compared with IgG-Dye + NIR group, ### means *P = 0.015673, αCD73-Dye + NIR group compared with αCD73-Dye group, one-way ANOVA with Tukey’s correction (i).
Extended Data Fig. 3 αCD73-Dye + NIR irradiation synergizes with αPD-1, promoting curative responses in the 4T1.2 tumour model.
(a) Diagram of the treatments. BALB/c mice bearing both orthotopic 4T1.2 tumor (5 × 105 4T1.2 tumor cells injection in the mammary gland) and lung metastasis tumors (1 × 105 tumor cells injection via tail vein) were treated on day 7 with control IgG, αPD-1, αCD73-Dye + αPD-1, αCD73-Dye + NIR, or αCD73-Dye + NIR + αPD-1 (Combo). NIR irradiation was given on the orthotopic tumor only (other parts of the mice were shielded from light). (b) Tumor growth curves (n = 5 mice/group) of the orthotopic 4T1.2 tumors after indicated treatments. (c) Some mice were euthanized on day 28 and representative lung pictures are shown. (d) Summarized lung weight of mice receiving indicated treatments (n = 5 biological replicates). (e) Surviving curves of 4T1.2 tumor-bearing mice. Representative results from one of two repeated experiments are shown (n = 10 mice/group). Data are mean ± SD. ****P < 0.0001, Combo group compared with IgG, αPD-1, αCD73-Dye + αPD-1, or αCD73-Dye + NIR group, one-way ANOVA with Tukey’s correction (d). ***P < 0.001, αCD73-Dye + NIR group compared with IgG, αPD-1, or αCD73-Dye + αPD-1 group; ****P < 0.0001, Combo group compared with IgG, αPD-1, αCD73-Dye + αPD-1, or αCD73-Dye + NIR group, survival analysis was conducted by log-rank test with holm test for multiple comparisons (e).
Extended Data Fig. 4 αCD73-Dye + NIR + αPD-1 combination treatment induced curative responses in the Pan02 tumor model.
(a) Diagram of the treatments. Pan02 tumor cells were s.c. injected on the left (2 × 106 Pan02 cells) and right (5 × 105 Pan02 cells) flanks of B6 mice, and treated on day 7 with control IgG, αPD-1, αCD73-Dye + αPD-1, αCD73-Dye + NIR, or αCD73-Dye + NIR + αPD-1 (Combo). NIR was performed on the left-side tumor only (right-side tumors were shielded from light). (b) Tumor growth curves of NIR-treated left tumors are shown (n = 5 mice/group). (c) Tumor growth curves of non-NIR-treated right tumors are shown (n = 5 mice/group). (d) Surviving curves of Pan02 tumor-bearing mice (n = 10 mice/group). (e) Tumor growth curves and (f) survival curves of Pan02 tumor-bearing mice treated with αCD73-Dye + NIR + αPD-1 combo therapy together with the depletion of NK cells, CD4 + T cells or CD8 + T cells. Representative results from one of two repeated experiments are shown. Data are mean ± SD. # means ****P < 0.0001, αCD73-Dye + NIR group compared with IgG, αPD-1, or αCD73-Dye + αPD-1 group; # means **P = 0.00592, Combo group compared with αCD73-Dye + NIR group, ## means **** P < 0.0001, Combo group compared with IgG, αPD-1, or αCD73-Dye + αPD-1 group, two-way ANOVA with Holm–Sidak test for multiple comparisons (c). ***P < 0.001, αCD73-Dye + NIR group compared with IgG, αPD-1, or αCD73-Dye + αPD-1 group, ****P < 0.0001, Combo group compared with IgG, αPD-1, αCD73-Dye + αPD-1, or αCD73-Dye + NIR group, survival analysis was conducted by log-rank test with holm test for multiple comparisons (d). ***P < 0.001, Combo + αCD8 group compared with Combo + IgG, Combo + αNK1.1, or Combo + αCD4 group, two-way ANOVA with Holm–Sidak test for multiple comparisons (e). ***P < 0.001, Combo + αCD8 group compared with Combo + IgG, Combo + αNK1.1, or Combo + αCD4 group, survival analysis was conducted by log-rank test with holm test for multiple comparisons (f).
Extended Data Fig. 5 Effects of αCD73-Dye + NIR + αPD-1 combination treatment in the EMT6CD73 KO tumor model.
(a) FACS analysis for surface expression of CD73 in EMT6 CD73 knockout (EMT6CD73 KO) cells. (b) EMT6CD73 KO cells were treated as indicated in vitro. The percentage of dead cells was determined by FACS after PI staining (n = 3 biological replicates). (c) BALB/c mice bearing both orthotopic EMT6CD73 KO tumor (5 × 105 EMT6CD73 KO tumor cells injection in the mammary gland) and lung metastasis tumors (1 × 105 EMT6CD73 KO tumor cells injection via tail vein) were treated on day 5 with control IgG, αPD-1, αCD73-Dye + αPD-1, αCD73-Dye + NIR, or αCD73-Dye + NIR + αPD-1 (Combo). NIR irradiation was given on the orthotopic tumor only (other parts of the mice were shielded from light). Mice survival curves (n = 9/αPD-1 group, n = 11/αCD73-Dye + NIR group, n = 12/ Combo group, n = 10/other groups) are shown. Representative results from one of two repeated experiments are shown. Data are mean ± SD. # mean **P = 0.00114439, Combo group compared with αCD73-Dye + NIR group; ## means ***P < 0.001, Combo group compared with IgG, αPD-1, or αCD73-Dye + αPD-1 group, survival analysis was conducted by log-rank test with holm test for multiple comparisons (c).
Extended Data Fig. 6 Effects of αCD73-Dye + NIR + αPD-1 combination therapy in human pancreatic cancer.
(a) CD73 protein levels in the HPA dataset. Most cancer tissues displayed strong to moderate membranous and cytoplasmic CD73 positivity. Lymphomas and testicular cancers showed weak positivity or were negative. (b) Cell number per 100 mg tumor tissue of indicated immune cell subsets were determined by FACS (n = 3). (c) FACS analysis for surface expression of CD73 or B7H3 on cells isolated from pancreatic tumor specimens. Representative data are shown. (d) Diagram of organotypic tumor spheroids (OTS), modified from a published study62. (e) Human PDAC OTS were treated as indicated ex vivo. Cell death was tested by Nexcelom ViaStain AO/PI staining Solution. Green represents live cells; red represents dead cells. Orange bar: 50 μM. Representative data and summarized results are shown, n = 8/group (2-3 OTS from each patient for each indicated treatment; and OTS from 3 patients were used). Data are mean ± SD. ***P < 0.001, αCD73-Dye + NIR + αPD-1 group compared with any other groups, one-way ANOVA with Tukey’s correction (e).
Supplementary information
Supplementary Information
Supplementary figures.
Source data
SD for Fig. 1
Source data.
SD for Fig. 3
Source data.
SD for Fig. 4
Source data.
SD for Fig. 5
Source data.
SD for Fig. 6
Source data.
SD for Fig. 7
Source data.
SD for ED Fig. 1
Source data.
SD for ED Fig. 2
Source data.
SD for ED Fig. 3
Source data.
SD for ED Fig. 4
Source data.
SD for ED Fig. 5
Source data.
SD for ED Fig. 6
Source data.
Rights and permissions
About this article
Cite this article
Xue, G., Wang, Z., Zheng, N. et al. Elimination of acquired resistance to PD-1 blockade via the concurrent depletion of tumour cells and immunosuppressive cells. Nat Biomed Eng 5, 1306–1319 (2021). https://doi.org/10.1038/s41551-021-00799-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00799-6
This article is cited by
-
Emerging biomaterials for tumor immunotherapy
Biomaterials Research (2023)
-
Nanosized drug delivery systems modulate the immunosuppressive microenvironment to improve cancer immunotherapy
Acta Pharmacologica Sinica (2022)