Abstract
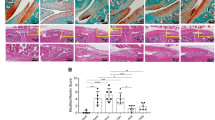
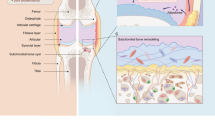
The progression of osteoarthritis is associated with inflammation triggered by the enzymatic degradation of extracellular matrix in injured cartilage. Here we show that a locally injected depot of nanoparticles functionalized with an antibody targeting type II collagen and carrying small interfering RNA targeting the matrix metalloproteinase 13 gene (Mmp13), which breaks down type II collagen, substantially reduced the expression of MMP13 and protected cartilage integrity and overall joint structure in acute and severe mouse models of post-traumatic osteoarthritis. MMP13 inhibition suppressed clusters of genes associated with tissue restructuring, angiogenesis, innate immune responses and proteolysis. We also show that intra-articular injections of the nanoparticles led to greater reductions in disease progression than either a single injection or weekly injections of the steroid methylprednisolone. Sustained drug retention by targeting collagen in the damaged extracellular matrix of osteoarthritic cartilage may also be an effective strategy for the treatment of osteoarthritis with other disease-modifying drugs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Raw and normalized nanoString datasets are available at the Gene Expression Omnibus under accession identifier GSE171031. The remaining raw and analysed datasets from the study are too large to be publicly shared, but they are available for research purposes from the corresponding author on reasonable request.
References
Loeser, R. F. Osteoarthritis year in review 2013: biology. Osteoarthr. Cartil. 21, 1436–1442 (2013).
Tanamas, S. et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Care Res. 61, 459–467 (2009).
Richette, P. et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann. Rheum. Dis. 70, 139–144 (2011).
Valdes, A. M. & Spector, T. D. Genetic epidemiology of hip and knee osteoarthritis. Nat. Rev. Rheumatol. 7, 23–32 (2011).
Issa, S. & Sharma, L. Epidemiology of osteoarthritis: an update. Curr. Rheumatol. Rep. 8, 7–15 (2006).
Lee, A. S. et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 527, 440–447 (2013).
Zhang, Y. & Jordan, J. M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 26, 355–369 (2010).
Brophy, R. H., Gray, B. L., Nunley, R. M., Barrack, R. L. & Clohisy, J. C. Total knee arthroplasty after previous knee surgery. J. Bone Jt. Surg. Am. 96A, 801–805 (2014).
Brown, T. D., Johnston, R. C., Saltzman, C. L., Marsh, J. L. & Buckwalter, J. A. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 20, 739–744 (2006).
Young, I. C. et al. A novel compressive stress-based osteoarthritis-like chondrocyte system. Exp. Biol. Med. 242, 1062–1071 (2017).
Martin, J. & Buckwalter, J. Post-traumatic osteoarthritis: the role of stress induced chondrocyte damage. Biorheology 43, 517–521 (2006).
McAlindon, T. E. et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 22, 363–388 (2014).
McAlindon, T. E. et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA 317, 1967–1975 (2017).
Wijn, S. R. W., Rovers, M. M., van Tienen, T. G. & Hannink, G. Intra-articular corticosteroid injections increase the risk of requiring knee arthroplasty. Bone Jt. J. 102-B, 586–592 (2020).
Wernecke, C., Braun, H. J. & Dragoo, J. L. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop. J. Sports Med. 3, 2325967115581163 (2015).
Wang, M. et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res. Ther. 15, R5 (2013).
Krzeski, P. et al. Development of musculoskeletal toxicity without clear benefit after administration of PG-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: a randomized, 12-month, double-blind, placebo-controlled study. Arthritis Res. Ther. 9, R109 (2007).
Molina, J. R. et al. A phase I and pharmacokinetic study of the selective, non-peptidic inhibitor of matrix metalloproteinase BAY 12-9566 in combination with etoposide and carboplatin. Anticancer Drugs 16, 997–1002 (2005).
Clutterbuck, A. L., Asplin, K. E., Harris, P., Allaway, D. & Mobasheri, A. Targeting matrix metalloproteinases in inflammatory conditions. Curr. Drug Targets 10, 1245–1254 (2009).
Liu, J. & Khalil, R. A. Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog. Mol. Biol. Transl. Sci. 148, 355–420 (2017).
Settle, S. et al. Cartilage degradation biomarkers predict efficacy of a novel, highly selective matrix metalloproteinase 13 inhibitor in a dog model of osteoarthritis: confirmation by multivariate analysis that modulation of type II collagen and aggrecan degradation peptides parallels pathologic changes. Arthritis Rheum. 62, 3006–3015 (2010).
Cai, H. et al. Assessment of the renal toxicity of novel anti-inflammatory compounds using cynomolgus monkey and human kidney cells. Toxicology 258, 56–63 (2009).
Sterner, B. et al. The effect of polymer size and charge of molecules on permeation through synovial membrane and accumulation in hyaline articular cartilage. Eur. J. Pharm. Biopharm. 101, 126–136 (2016).
Larsen, C. et al. Intra-articular depot formulation principles: role in the management of postoperative pain and arthritic disorders. J. Pharm. Sci. 97, 4622–4654 (2008).
Evans, C. H., Kraus, V. B. & Setton, L. A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 10, 11–22 (2013).
Simkin, P. A. Synovial perfusion and synovial fluid solutes. Ann. Rheum. Dis. 54, 424–428 (1995).
Rothenfluh, D. A., Bermudez, H., O’Neil, C. P. & Hubbell, J. A. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat. Mater. 7, 248–254 (2008).
Zhou, F. et al. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 63, 64–75 (2017).
Hayder, M. et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci. Transl. Med. 3, 81ra35 (2011).
Chen, K. & Chen, X. Integrin targeted delivery of chemotherapeutics. Theranostics 1, 189–200 (2011).
Hoy, S. M. Patisiran: first global approval. Drugs 78, 1625–1631 (2018).
de Paula Brandão, P. R., Titze-de-Almeida, S. S. & Titze-de-Almeida, R. Leading RNA interference therapeutics part 2: silencing delta-aminolevulinic acid synthase 1, with a focus on givosiran. Mol. Diagnosis Ther. 24, 61–68 (2019).
Nelson, C. E. et al. Balancing cationic and hydrophobic content of PEGylated siRNA polyplexes enhances endosome escape, stability, blood circulation time, and bioactivity in vivo. ACS Nano 7, 8870–8880 (2013).
Beavers, K. R., Nelson, C. E. & Duvall, C. L. MiRNA inhibition in tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 88, 123–137 (2015).
Sarett, S. M. et al. Hydrophobic interactions between polymeric carrier and palmitic acid-conjugated siRNA improve PEGylated polyplex stability and enhance in vivo pharmacokinetics and tumor gene silencing. Biomaterials 97, 122–132 (2016).
Jackson, M. A. et al. Zwitterionic nanocarrier surface chemistry improves siRNA tumor delivery and silencing activity relative to polyethylene glycol. ACS Nano 11, 5680–5696 (2017).
Ruan, M. Z. et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci. Transl. Med. 5, 176ra134 (2013).
Cho, H., Pinkhassik, E., David, V., Stuart, J. M. & Hasty, K. A. Detection of early cartilage damage using targeted nanosomes in a post-traumatic osteoarthritis mouse model. Nanomed. Nanotechnol. Biol. Med. 11, 939–946 (2015).
Werfel, T. A. et al. Selective mTORC2 inhibitor therapeutically blocks breast cancer cell growth and survival. Cancer Res. 78, 1845–1858 (2018).
Werfel, T. A. et al. Combinatorial optimization of PEG architecture and hydrophobic content improves ternary siRNA polyplex stability, pharmacokinetics, and potency in vivo. J. Control. Release 255, 12–26 (2017).
Kilchrist, K. V. et al. Gal8 visualization of endosome disruption predicts carrier-mediated biologic drug intracellular bioavailability. ACS Nano 13, 1136–1152 (2019).
Jackson, M. A. et al. Dual carrier–cargo hydrophobization and charge ratio optimization improve the systemic circulation and safety of zwitterionic nano-polyplexes. Biomaterials 192, 245–259 (2019).
Griffin, D. J. et al. Effects of enzymatic treatments on the depth-dependent viscoelastic shear properties of articular cartilage. J. Orthop. Res. 32, 1652–1657 (2014).
Cho, H. et al. Theranostic immunoliposomes for osteoarthritis. Nanomedicine 10, 619–627 (2014).
Jasin, H. E., Noyori, K., Takagi, T. & Taurog, J. D. Characteristics of anti-type II collagen antibody binding to articular cartilage. Arthritis Rheum. 36, 651–659 (1993).
Poulet, B., Hamilton, R. W., Shefelbine, S. & Pitsillides, A. A. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 63, 137–147 (2011).
Ko, F. C. et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 65, 1569–1578 (2013).
Scanzello, C. R. & Goldring, S. R. The role of synovitis in osteoarthritis pathogenesis. Bone 51, 249–257 (2012).
Goldring, M. B. Articular cartilage degradation in osteoarthritis. HSS J. 8, 7–9 (2012).
Glasson, S. S., Chambers, M. G., Van Den Berg, W. B. & Little, C. B. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 18, S17–S23 (2010).
Eichaker, L. R., Cho, H., Duvall, C. L., Werfel, T. A. & Hasty, K. A. Future nanomedicine for the diagnosis and treatment of osteoarthritis. Nanomedicine 9, 2203–2215 (2014).
O’Grady, K. P. et al. Drug-free ROS sponge polymeric microspheres reduce tissue damage from ischemic and mechanical injury. ACS Biomater. Sci. Eng. 4, 1251–1264 (2017).
Sun, Y. & Mauerhan, D. R. Meniscal calcification, pathogenesis and implications. Curr. Opin. Rheumatol. 24, 152–157 (2012).
Blom, A. B. et al. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum. 56, 147–157 (2007).
Hügle, T. & Geurts, J. What drives osteoarthritis?—Synovial versus subchondral bone pathology. Rheumatology 56, 1461–1471 (2016).
Goldring, S. R. Alterations in periarticular bone and cross talk between subchondral bone and articular cartilage in osteoarthritis. Ther. Adv. Musculoskelet. Dis. 4, 249–258 (2012).
Boileau, C., Tat, S. K., Pelletier, J. P., Cheng, S. & Martel-Pelletier, J. Diacerein inhibits the synthesis of resorptive enzymes and reduces osteoclastic differentiation/survival in osteoarthritic subchondral bone: a possible mechanism for a protective effect against subchondral bone remodelling. Arthritis Res. Ther. 10, R71 (2008).
Punzi, L. et al. Post-traumatic arthritis: overview on pathogenic mechanisms and role of inflammation. RMD Open 2, e000279 (2016).
Christiansen, B. A. et al. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthr. Cartil. 23, 1627–1638 (2015).
Almasry, S. M., Soliman, H. M., El-Tarhouny, S. A., Algaidi, S. A. & Ragab, E. M. Platelet rich plasma enhances the immunohistochemical expression of platelet derived growth factor and vascular endothelial growth factor in the synovium of the meniscectomized rat models of osteoarthritis. Ann. Anat. 197, 38–49 (2015).
Solomon, L. A., Bérubé, N. G. & Beier, F. Transcriptional regulators of chondrocyte hypertrophy. Birth Defects Res. C 84, 123–130 (2008).
Zhai, G., Doré, J. & Rahman, P. TGF-β signal transduction pathways and osteoarthritis. Rheumatol. Int. 35, 1283–1292 (2015).
Shen, J., Li, S. & Chen, D. TGF-β signaling and the development of osteoarthritis. Bone Res. https://doi.org/10.1038/boneres.2014.2 (2014).
Lee, Y. H. et al. Enzyme-crosslinked gene-activated matrix for the induction of mesenchymal stem cells in osteochondral tissue regeneration. Acta Biomater. 63, 210–226 (2017).
John, T., Stahel, P. F., Morgan, S. J. & Schulze-Tanzil, G. Impact of the complement cascade on posttraumatic cartilage inflammation and degradation. Histol. Histopathol. 22, 781–790 (2007).
Silawal, S., Triebel, J., Bertsch, T. & Schulze-Tanzil, G. Osteoarthritis and the complement cascade. Clin. Med. Insights Arthritis Musculoskelet. Disord. 11, 1179544117751430 (2018).
Lubbers, R., van Essen, M. F., van Kooten, C. & Trouw, L. A. Production of complement components by cells of the immune system. Clin. Exp. Immunol. 188, 183–194 (2017).
Takahashi, N. et al. Elucidation of IL-1/TGF-β interactions in mouse chondrocyte cell line by genome-wide gene expression. Osteoarthr. Cartil. 13, 426–438 (2005).
Klein-Wieringa, I. R. et al. Inflammatory cells in patients with endstage knee osteoarthritis: a comparison between the synovium and the infrapatellar fat pad. J. Rheumatol. 43, 771–778 (2016).
Wu, C. L., Harasymowicz, N. S., Klimak, M. A., Collins, K. H. & Guilak, F. The role of macrophages in osteoarthritis and cartilage repair. Osteoarthr. Cartil. https://doi.org/10.1016/j.joca.2019.12.007 (2020).
Mazur, C. M. et al. Osteocyte dysfunction promotes osteoarthritis through MMP13-dependent suppression of subchondral bone homeostasis. Bone Res. 7, 34 (2019).
Goldring, S. R. & Goldring, M. B. in Kelley’s Textbook of Rheumatology 1–19.e16 (Elsevier, 2017).
Garg, N., Perry, L. & Deodhar, A. Intra-articular and soft tissue injections, a systematic review of relative efficacy of various corticosteroids. Clin. Rheumatol. 33, 1695–1706 (2014).
Cho, H., Walker, A., Williams, J. & Hasty, K. A. Study of osteoarthritis treatment with anti-inflammatory drugs: cyclooxygenase-2 inhibitor and steroids. BioMed. Res. Int. 2015, 595273 (2015).
Hoshyar, N., Gray, S., Han, H. & Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomed. 11, 673–692 (2016).
Terato, K. et al. Induction of arthritis with monoclonal antibodies to collagen. J. Immunol. 148, 2103–2108 (1992).
Evans, B. C. et al. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp. 73, e50166 (2013).
Polderman, J. A. et al. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst. Rev. 11, CD011940 (2018).
Orak, M. M. et al. Comparison of the effects of chronic intra-articular administration of tenoxicam, diclofenac, and methylprednisolone in healthy rats. Acta Orthop. Traumatol. Turc. 49, 438–446 (2015).
Bolon, B. et al. Rodent preclinical models for developing novel antiarthritic molecules: comparative biology and preferred methods for evaluating efficacy. J. Biomed. Biotechnol. 2011, 569068 (2011).
Aigner, T. & Söder, S. Histopathologische Begutachtung der Gelenkdegeneration. Der. Pathol. 27, 431–438 (2006).
Acknowledgements
The authors acknowledge the assistance of the Vanderbilt TPSR. The TPSR is supported by National Cancer Institute/National Insitutes for Health (NIH) Cancer Center Support Grant 2P30 CA068485-14. Dynamic light scattering was conducted at the Vanderbilt Institute of Nanoscale Sciences and Engineering. Bone analysis by microCT was supported in part by the NIH (S10RR027631-01). We thank C. B. Wiese, J. R. Johnson and R. Mernaugh for technical assistance. The VANTAGE core performed nanoString QC and hybridization, and is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485), the Vanderbilt Vision Center (P30 EY08126) and NIH/National Centre for Research Resources (G20 RR030956). We thank the Department of Defense (DOD CDMRP OR130302), NIH (NIH R01 CA224241 and NIH R01 EB019409), NIH (NIGMS T32GM007347), the Veterans Association Merit Award BX004151, the National Science Foundation Graduate Research Fellowship Program (NSF GRF 2016212929), the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Rheumatology Research Foundation (RRF) for support.
Author information
Authors and Affiliations
Contributions
S.K.B., C.L.D., K.A.H., L.J.C. and J.M.C. designed the project and experiments. S.K.B. synthesized the polymers and conjugates and formulated nanoparticles for all experiments. S.K.B. and F.Y. conducted all animal experiments. S.K.B., M.A.J. and D.D.L. performed nanoparticle characterization. S.K.B., F.Y., J.M.C. and D.D.L. imaged and collected tissues. L.E.H. and H.C. conducted histology and immunohistochemistry, with L.E.H. performing blinded scoring of histology. S.K.B., C.L.D. and J.M.C. wrote the manuscript. All authors reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks Fergal O’Brien, Ahuva Nissim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Rights and permissions
About this article
Cite this article
Bedingfield, S.K., Colazo, J.M., Yu, F. et al. Amelioration of post-traumatic osteoarthritis via nanoparticle depots delivering small interfering RNA to damaged cartilage. Nat Biomed Eng 5, 1069–1083 (2021). https://doi.org/10.1038/s41551-021-00780-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00780-3
This article is cited by
-
Engineering approaches for RNA-based and cell-based osteoarthritis therapies
Nature Reviews Rheumatology (2024)
-
Molecular co-assembled strategy tuning protein conformation for cartilage regeneration
Nature Communications (2024)
-
Tribological behavior of shape-specific microplate-enriched synovial fluids on a linear two-axis tribometer
Friction (2024)
-
Intra-articular delivery of geraniol encapsulated by pH/redox-responsive nanogel ameliorates osteoarthritis by regulating oxidative stress and inflammation
Journal of Molecular Histology (2023)
-
Modification of mesenchymal stem cells for cartilage-targeted therapy
Journal of Translational Medicine (2022)