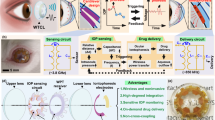
Abstract
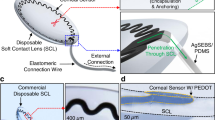
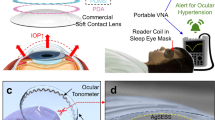
Continuous detection of raised intraocular pressure (IOP) could benefit the monitoring of patients with glaucoma. Current contact lenses with embedded sensors for measuring IOP are rigid, bulky, partially block vision or are insufficiently sensitive. Here, we report the design and testing in volunteers of a soft and transparent contact lens for the quantitative monitoring of IOP in real time using a smartphone. The contact lens incorporates a strain sensor, a wireless antenna, capacitors, resistors, stretchable metal interconnects and an integrated circuit for wireless communication. In rabbits, the lens provided measurements that match those of a commercial tonometer. In ten human participants, the lens proved to be safe, and reliably provided accurate quantitative measurements of IOP without inducing inflammation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The rabbit in vivo data are available at Figshare (https://doi.org/10.6084/m9.figshare.13289342)52. The human data are available within the paper and its Supplementary Information. The raw and analysed datasets generated for the studies shown in Figs. 1–3 are available for research purposes from the corresponding authors on reasonable request.
References
Yang, X. et al. Bioinspired neuron-like electronics. Nat. Mater. 18, 510–517 (2019).
Xu, S. et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 344, 70–74 (2014).
Dai, X., Zhou, W., Gao, T., Liu, J. & Lieber, C. M. Three-dimensional mapping and regulation of action potential propagation in nanoelectronics-innervated tissues. Nat. Nanotechnol. 11, 776–782 (2016).
Zhang, K. et al. Origami silicon optoelectronics for hemispherical electronic eye systems. Nat. Commun. 8, 1782 (2017).
Anwar, T. et al. p38-mediated phosphorylation at T367 induces EZH2 cytoplasmic localization to promote breast cancer metastasis. Nat. Commun. 9, 2801 (2018).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Kim, D.-H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Lipani, L. et al. Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform. Nat. Nanotechnol. 13, 504–511 (2018).
Chen, G.-Z., Chan, I.-S. & Lam, D. C. C. Capacitive contact lens sensor for continuous non-invasive intraocular pressure monitoring. Sens. Actuators A 203, 112–118 (2013).
Casson, R. J., Chidlow, G., Wood, J. P., Crowston, J. G. & Goldberg, I. Definition of glaucoma: clinical and experimental concepts. Clin. Exp. Ophthalmol. 40, 341–349 (2012).
Moraes, C. G. V. D. et al. Risk factors for visual field progression in treated glaucoma. Arch. Ophthalmol. 129, 562–568 (2011).
Renard, E. et al. Twenty-four hour (nyctohemeral) rhythm of intraocular pressure and ocular perfusion pressure in normal-tension glaucoma. Invest. Ophthalmol. Vis. Sci. 51, 882–889 (2010).
Ku, M. et al. Smart, soft contact lens for wireless immunosensing of cortisol. Sci. Adv. 6, eabb2891 (2020).
Moses, R. A. The Goldmann applanation tonometer. Am. J. Ophthalmol. 46, 865–869 (1958).
Muir, K. W. Home tonometry-can we? should we? JAMA Ophthalmol. 135, 1036 (2017).
Hom, M. & Bruce, A. Manual of Contact Lens Prescribing and Fitting with CD-ROM 3rd edn (Butterworth-Heinemann, 2006).
Flatau, A. et al. Measured changes in limbal strain during simulated sleep in face down position using an instrumented contact lens in healthy adults and adults with glaucoma. JAMA Ophthalmol. 134, 375–382 (2016).
Leonardi, M., Pitchon, E. M., Bertsch, A., Renaud, P. & Mermoud, A. Wireless contact lens sensor for intraocular pressure monitoring: assessment on enucleated pig eyes. Acta Ophthalmol. 87, 433–437 (2009).
Liao, Y. T., Yao, H., Lingley, A., Parviz, B. & Otis, B. P. A 3-μW CMOS glucose sensor for wireless contact-lens tear glucose monitoring. IEEE J. Solid State Circuits 47, 335–344 (2012).
Piso, D., Veiga-Crespo, P. & Vecino, E. Modern monitoring intraocular pressure sensing devices based on application specific integrated circuits. J. Biomater. Nanobiotechnol 3, 301–309 (2012).
Pandey, J. et al. A fully integrated RF-powered contact lens with a single element display. IEEE Trans. Biomed. Circuits Syst. 4, 454–461 (2010).
Kim, J. et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 8, 14997 (2017).
Dunbar, G. E., Shen, B. Y. & Aref, A. A. The sensimed triggerfish contact lens sensor: efficiency, safety, and patient perspectives. Clin. Ophthalmol. 11, 875–882 (2017).
Park, J. et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 4, eaap9841 (2018).
Romeo, A., Liu, Q., Suo, Z. & Lacour, S. P. Elastomeric substrates with embedded stiff platforms for stretchable electronics. Appl. Phys. Lett. 102, 131904 (2013).
Robinson, A., Aziz, A., Liu, Q., Suo, Z. & Lacour, S. P. Hybrid stretchable circuits on silicone substrate. J. Appl. Phys. 115, 143511 (2014).
Hjortdal, J. Ø. & Jensen, P. K. In vitro measurement of corneal strain, thickness, and curvature using digital image processing. Acta Ophthalmol. Scand. 73, 5–11 (1995).
Leonardi, M., Leuenberger, P., Bertrand, D., Bertsch, A. & Renaud, P. First steps toward noninvasive intraocular pressure monitoring with a sensing contact lens. Invest. Ophthalmol. Vis. Sci. 45, 3113–3117 (2004).
Douthwaite, W. A. & Lam, A. K. C. The effect of an artificially elevated intraocular pressure on the central corneal curvature. Ophthalmic Physiol. Opt. 17, 18–24 (1997).
Tadakaluru, S., Thongwusan, W. & Singjai, P. Stretchable and flexible high-strain sensors made using carbon nanotubes and graphite films on natural rubber. Sensors 14, 868–876 (2014).
Yamada, T. et al. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 6, 296–301 (2011).
Amjadi, M., Pichitpajongkit, A., Lee, S., Ryu, S. & Park, I. Highly stretchable and sensitive strain sensor based on silver nanowire-elastomer nanocomposite. ACS Nano 8, 5154–5163 (2014).
Gong, S. et al. Highly stretchy black gold e-skin nanopatches as highly sensitive wearable biomedical sensors. Adv. Electron. Mater. 1, 1400063 (2015).
Yang, S. & Lu, N. Gauge factor and stretchability of silicon-on-polymer strain gauges. Sensors 13, 8577–8594 (2013).
Kim, D.-H. et al. Electronic sensor and actuator webs for large-area complex geometry cardiac mapping and therapy. Proc. Natl Acad. Sci. USA 109, 19910–19915 (2012).
Hoffmann, E. M., Grus, F.-H. & Pfeiffer, N. Intraocular pressure and ocular pulse amplitude using dynamic contour tonometry and contact lens tonometry. BMC Ophthalmol. 4, 4 (2004).
Lee, J. O. et al. A microscale optical implant for continuous in vivo monitoring of intraocular pressure. Microsyst. Nanoeng. 3, 17057 (2017).
Kim, K. H., Lee, J. O., Du, J., Sretavan, D. & Choo, H. Real-time in vivo intraocular pressure monitoring using an optomechanical implant and an artificial neural network. IEEE Sens. J. 17, 7394–7404 (2018).
Kim, J. et al. Epidermal electronics with advanced capabilities in near-field communication. Small 11, 906–912 (2015).
Kim, J. et al. Miniaturized flexible electronic systems with wireless power and near-field communication capabilities. Adv. Funct. Mater. 25, 4761–4767 (2015).
Tao, H. et al. Silk‐based conformal, adhesive, edible food sensors. Adv. Mater. 24, 1067–1072 (2012).
Cheng, S. & Wu, Z. Microfluidic, reversibly stretchable, large-area wireless strain sensor. Adv. Funct. Mater. 21, 2282–2290 (2011).
Kim, J. et al. Highly transparent and stretchable field-effect transistor sensors using graphene–nanowire hybrid nanostructures. Adv. Mater. 27, 3292–3297 (2015).
Mujal, J. et al. Inkjet printed antennas for NFC systems. In Proc. 17th IEEE International Conference on Electronics, Circuits and Systems 1220–1223 (IEEE, 2010).
Li, S., Sun, F., An, D. & He, S. Increasing efficiency of a wireless energy transfer system by spatial translational transformation. IEEE Trans. Power Electron. 99, 3325–3332 (2017).
Ladd, C., So, J.-H., Muth, J. & Dickey, M. D. 3D printing of free standing liquid metal microstructures. Adv. Mater. 25, 5081–5085 (2013).
IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz IEEE Std C95.1-2005 (Revision of IEEE Std C95.1-1991), 1–238 (IEEE, 2006).
Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices ASTM F1980‐07 (American Society for Testing Materials, 2011).
Efron, N. Grading scales for contact lens complications. Ophthalmic Physiol. Opt. 18, 182–186 (1998).
First, P. G. et al. The influence of soft contact lenses on the intraocular pressure measurement. Eye 26, 278–282 (2012).
Realini, T., Weinreb, R. N. & Wisniewski, S. R. Diurnal intraocular pressure patterns are not repeatable in the short-term in healthy individuals. Ophthalmology 117, 1700–1704 (2010).
Kim, J. et al. Dataset for ‘A soft and transparent contact lens for the wireless quantitative monitoring of intraocular pressure’. Figshare https://doi.org/10.6084/m9.figshare.13289342 (2020).
Acknowledgements
This work was supported by the Ministry of Science & ICT (MSIT) and the Ministry of Trade, Industry and Energy (MOTIE) of Korea through the National Research Foundation (2019R1A2B5B03069358 and 2016R1A5A1009926), the Bio & Medical Technology Development Program (2018M3A9F1021649), the Nano Material Technology Development Program (2016M3A7B4910635) and the Technology Innovation Program (20010366 and 20013621, Center for Super Critical Material Industrial Technology). We also acknowledge financial support from the Institute for Basic Science (IBS-R026-D1), the Research Program (2019-22-0228) and the Post Doc Researcher Supporting program of 2019 (2019-12-0010) funded by Yonsei University.
Author information
Authors and Affiliations
Contributions
Joohee Kim and J.P. carried out the experiments, analysed the data and wrote the manuscript. Y.-G.P. performed the experiments related to the printing of stretchable 3D interconnects. E.C. and M.K. performed the fabrication of contact lenses. H.S.A. participated in the design of stretchable antennas. K.-P.L. and M.-I.H. participated in the in vivo experiments. Junmo Kim and T.-S.K. performed the DIC experiments. D.W.K., H.K.K. and J.-U.P. oversaw all of the research phases and revised the manuscript. All of the authors discussed and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–30 and Table 1, and captions for Supplementary Videos 1–3.
Supplementary Dataset
IOP data for the ten human volunteers.
Supplementary Video 1
In vivo trial of the soft contact lens for recording IOP.
Supplementary Video 2
In vivo test conducted using a live rabbit for monitoring the generation of heat during the wireless operation of the contact lens.
Supplementary Video 3
Human pilot trial of the soft contact lens.
Rights and permissions
About this article
Cite this article
Kim, J., Park, J., Park, YG. et al. A soft and transparent contact lens for the wireless quantitative monitoring of intraocular pressure. Nat Biomed Eng 5, 772–782 (2021). https://doi.org/10.1038/s41551-021-00719-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00719-8
This article is cited by
-
Material and structural considerations for high-performance electrodes for wearable skin devices
Communications Materials (2024)
-
In-depth correlation analysis between tear glucose and blood glucose using a wireless smart contact lens
Nature Communications (2024)
-
In-vivo integration of soft neural probes through high-resolution printing of liquid electronics on the cranium
Nature Communications (2024)
-
Recent advancements in nanomaterial-laden contact lenses for diagnosis and treatment of glaucoma, review and update
Journal of Nanobiotechnology (2023)
-
An artificially-intelligent cornea with tactile sensation enables sensory expansion and interaction
Nature Communications (2023)