Abstract
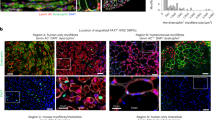
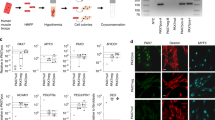
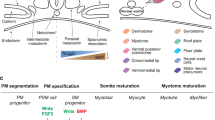
Because post-mortem human skeletal muscle is not viable, autologous muscle grafts are typically required in tissue reconstruction after muscle loss due to disease or injury. However, the use of autologous tissue often leads to donor-site morbidity. Here, we show that intraspecies and interspecies chimaeric pig embryos lacking native skeletal muscle can be produced by deleting the MYF5, MYOD and MYF6 genes in the embryos via CRISPR, followed by somatic-cell nuclear transfer and the delivery of exogenous cells (porcine blastomeres or human induced pluripotent stem cells) via blastocyst complementation. The generated intraspecies chimaeras were viable and displayed normal histology, morphology and function. Human:pig chimaeras generated with TP53-null human induced pluripotent stem cells led to higher chimaerism efficiency, with embryos collected at embryonic days 20 and 27 containing humanized muscle, as confirmed by immunohistochemical and molecular analyses. Human:pig chimaeras may facilitate the production of exogenic organs for research and xenotransplantation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The single-cell RNA-seq data from WT porcine morula and blastocyst embryos have been deposited in the NCBI Sequence Read Archive (SRA) database, under project accession no. PRJNA509275. All unique materials used are readily available from the authors or from commercial sources (Supplementary Tables 1–3). Gene-edited primary cell lines are available yet limited in number because of supply constraints.
References
Corona, B. T., Rivera, J. C., Owens, J. G., Wenke, J. C. & Rathbone, C. R. Volumetric muscle loss leads to permanent disability following extremity trauma. J. Rehabil. Res. Dev. 52, 785–792 (2015).
Pollot, B. E. & Corona, B. T. Volumetric muscle loss. Methods Mol. Biol. 1460, 19–31 (2016).
Greising, S. M. et al. Unwavering pathobiology of volumetric muscle loss injury. Sci. Rep. 7, 13179 (2017).
Grogan, B. F., Hsu, J. R. & Skeletal Trauma Research Consortium. Volumetric muscle loss. J. Am. Acad. Orthop. Surg. 19, S35–S37 (2011).
Kim, G. A. et al. Generation by somatic cell nuclear transfer of GGTA1 knockout pigs expressing soluble human TNFRI-Fc and human HO-1. Transgenic Res. 28, 91–102 (2019).
Liu, Z. et al. Cloning of a gene-edited macaque monkey by somatic cell nuclear transfer. Natl Sci. Rev. 6, 101–108 (2019).
Matsunari, H. et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc. Natl Acad. Sci. USA 110, 4557–4562 (2013).
Zhang, H. et al. Rescuing ocular development in an anophthalmic pig by blastocyst complementation. EMBO Mol. Med. 10, e8861 (2018).
Wu, J. et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell 168, 473–486 (2017).
Braun, T., Rudnicki, M. A., Arnold, H. H. & Jaenisch, R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71, 369–382 (1992).
Rawls, A. et al. Myogenin’s functions do not overlap with those of MyoD or Myf-5 during mouse embryogenesis. Dev. Biol. 172, 37–50 (1995).
Rudnicki, M. A., Braun, T., Hinuma, S. & Jaenisch, R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell 71, 383–390 (1992).
Rudnicki, M. A. et al. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75, 1351–1359 (1993).
Kassar-Duchossoy, L. et al. Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature 431, 466–471 (2004).
Ward, C. L. et al. Autologous minced muscle grafts improve muscle strength in a porcine model of volumetric muscle loss injury. J. Orthop. Trauma 30, e396–e403 (2016).
Petropoulos, S. et al. Single-cell RNA-seq reveals lineage and X chromosome dynamics in human preimplantation embryos. Cell 165, 1012–1026 (2016).
White, J. D., Rachel, C., Vermeulen, R., Davies, M. & Grounds, M. D. The role of p53 in vivo during skeletal muscle post-natal development and regeneration: studies in p53 knockout mice. Int. J. Dev. Biol. 46, 577–582 (2002).
Chen, G. et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 8, 424–429 (2011).
Cerbini, T. et al. Transcription activator-like effector nuclease (TALEN)-mediated CLYBL targeting enables enhanced transgene expression and one-step generation of dual reporter human induced pluripotent stem cell (iPSC) and neural stem cell (NSC) lines. PLoS ONE 10, e0116032 (2015).
Sakuma, T., Nishikawa, A., Kume, S., Chayama, K. & Yamamoto, T. Multiplex genome engineering in human cells using all-in-one CRISPR/Cas9 vector system. Sci. Rep. 4, 5400 (2014).
Koyano-Nakagawa, N. et al. Feedback mechanisms regulate Ets variant 2 (Etv2) gene expression and hematoendothelial lineages. J. Biol. Chem. 290, 28107–28119 (2015).
Whitworth, K. M. et al. Use of the CRISPR/Cas9 system to produce genetically engineered pigs from in vitro-derived oocytes and embryos. Biol. Reprod. 91, 78 (2014).
Das, S. et al. Generation of human endothelium in pig embryos deficient in ETV2. Nat. Biotechnol. 38, 297–302 (2020).
Maeng, G. et al. ETV2-null porcine embryos survive to post-implantation following incomplete enucleation. Reproduction 159, 539–547 (2020).
Lai, L. & Prather, R. S. Production of cloned pigs by using somatic cells as donors. Cloning Stem Cells 5, 233–241 (2003).
Koyano-Nakagawa, N. et al. Etv2 is expressed in the yolk sac hematopoietic and endothelial progenitors and regulates Lmo2 gene expression. Stem Cells 30, 1611–1623 (2012).
Rasmussen, T. L. et al. ER71 directs mesodermal fate decisions during embryogenesis. Development 138, 4801–4812 (2011).
Garry, M. G., Miller, K. E. & Seybold, V. S. Lumbar dorsal root ganglia of the cat: a quantitative study of peptide immunoreactivity and cell size. J. Comp. Neurol. 284, 36–47 (1989).
Romero, M. I., Rangappa, N., Garry, M. G. & Smith, G. M. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J. Neurosci. 21, 8408–8416 (2001).
Acknowledgements
This work was supported by a grant from Regenerative Minnesota Medicine (RMM; D.J.G.) and from the US Department of Defense (M.G.G.). We acknowledge NorthStar Genomics, DeSoto Biosciences, Recombinetics, MOFA Global, the University of Minnesota Genomics Center and the University of Minnesota Imaging Center for their technical assistance. We acknowledge the Cytogenomics Core at the University of Minnesota for karyotyping services. No NIH or RMM funding was used for the human chimaera portions of this study. We thank J. Hannah (Weizmann Institute of Science) for helpful discussions throughout the course of the studies. We also thank R. Prather and the National Swine Resource and Research Center at the University of Missouri for consultation (U42 OD011140).
Author information
Authors and Affiliations
Contributions
M.G.G. and D.J.G. conceived the project. G.M., S.D., D.J.G. and M.G.G. wrote the manuscript. G.M., S.D., S.K., B.N.S., D.M., S.M.G., J.R.S., E.S., W.G., C.V.W. and O.G. performed experiments and analysed the data. M.G.G. and D.J.G. supervised the project. All authors commented on and edited the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
D.J.G. and M.G.G. are co-founders of NorthStar Genomics. All other authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Rights and permissions
About this article
Cite this article
Maeng, G., Das, S., Greising, S.M. et al. Humanized skeletal muscle in MYF5/MYOD/MYF6-null pig embryos. Nat Biomed Eng 5, 805–814 (2021). https://doi.org/10.1038/s41551-021-00693-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00693-1
This article is cited by
-
Humanizing pig kidneys via chimeric complementation
Cell Research (2023)
-
Why it is important to study human–monkey embryonic chimeras in a dish
Nature Methods (2022)
-
New concepts for generating interspecies chimeras using human pluripotent stem cells
Protein & Cell (2022)