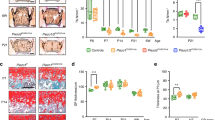
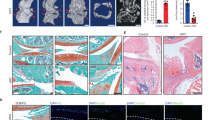
Abstract
Changes in the composition and viscoelasticity of the extracellular matrix in load-bearing cartilage influence the proliferation and phenotypes of chondrocytes, and are associated with osteoarthritis. However, the underlying molecular mechanism is unknown. Here we show that the viscoelasticity of alginate hydrogels regulates cellular volume in healthy human chondrocytes (with faster stress relaxation allowing cell expansion and slower stress relaxation restricting it) but not in osteoarthritic chondrocytes. Cellular volume regulation in healthy chondrocytes was associated with changes in anabolic gene expression, in the secretion of multiple pro-inflammatory cytokines, and in the modulation of intracellular calcium regulated by the ion-channel protein transient receptor potential cation channel subfamily V member 4 (TRPV4), which controls the phosphorylation of glycogen synthase kinase 3β (GSK3β), an enzyme with pleiotropic effects in osteoarthritis. A dysfunctional TRPV4–GSK3β pathway in osteoarthritic chondrocytes rendered the cells unable to respond to environmental changes in viscoelasticity. Our findings suggest strategies for restoring chondrocyte homeostasis in osteoarthritis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets are available in figshare with the identifier https://doi.org/10.6084/m9.figshare.13184909 (ref. 76).
References
Glyn-Jones, S. et al. Osteoarthritis. Lancet 386, 376–387 (2015).
Martel-Pelletier, J. et al. Osteoarthritis. Nat. Rev. Dis. Prim. 2, 16072 (2016).
Vincent, T. L. Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr. Opin. Pharmacol. 13, 449–454 (2013).
Kim, Y. J., Bonassar, L. J. & Grodzinsky, A. J. The role of cartilage streaming potential, fluid flow and pressure in the stimulation of chondrocyte biosynthesis during dynamic compression. J. Biomech. 28, 1055–1066 (1995).
Gu, W. Y., Lai, W. M. & Mow, V. C. Transport of fluid and ions through a porous-permeable charged-hydrated tissue, and streaming potential data on normal bovine articular cartilage. J. Biomech. 26, 709–723 (1993).
Buschmann, M. D., Gluzband, Y. A., Grodzinsky, A. J. & Hunziker, E. B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J. Cell Sci. 108, 1497–1508 (1995).
Guilak, F. Biomechanical factors in osteoarthritis. Best. Pract. Res. Clin. Rheumatol. 25, 815–823 (2011).
Maldonado, M. & Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed. Res. Int. 2013, 284873 (2013).
Goldring, S. R. & Goldring, M. B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage–bone crosstalk. Nat. Rev. Rheumatol. 12, 632–644 (2016).
Mow, V. C., Kuei, S. C., Lai, W. M. & Armstrong, C. G. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J. Biomech. Eng. 102, 73–84 (1980).
Nia, H. T., Han, L., Li, Y., Ortiz, C. & Grodzinsky, A. Poroelasticity of cartilage at the nanoscale. Biophys. J. 101, 2304–2313 (2011).
Mouw, J. K., Case, N. D., Guldberg, R. E., Plaas, A. H. K. & Levenston, M. E. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthr. Cartil. 13, 828–836 (2005).
Smeriglio, P., Lai, J. H., Yang, F. & Bhutani, N. 3D hydrogel scaffolds for articular chondrocyte culture and cartilage generation. J. Vis. Exp. 104, 53085 (2015).
Jutila, A. A., Zignego, D. L., Schell, W. J. & June, R. K. Encapsulation of chondrocytes in high-stiffness agarose microenvironments for in vitro modeling of osteoarthritis mechanotransduction. Ann. Biomed. Eng. 43, 1132–1144 (2015).
Guo, J. F., Jourdian, G. W. & MacCallum, D. K. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect. Tissue Res. 19, 277–297 (1989).
Genes, N. G., Rowley, J. A., Mooney, D. J. & Bonassar, L. J. Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Arch. Biochem. Biophys. 422, 161–167 (2004).
Degala, S., Zipfel, W. R. & Bonassar, L. J. Chondrocyte calcium signaling in response to fluid flow is regulated by matrix adhesion in 3-D alginate scaffolds. Arch. Biochem. Biophys. 505, 112–117 (2011).
Tan, H., Chu, C. R., Payne, K. A. & Marra, K. G. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 30, 2499–2506 (2009).
Cheng, H.-W., Tsui, Y.-K., Cheung, K. M. C., Chan, D. & Chan, B. P. Decellularization of chondrocyte-encapsulated collagen microspheres: a three-dimensional model to study the effects of acellular matrix on stem cell fate. Tissue Eng. C 15, 697–706 (2009).
Conrad, B., Han, L.-H. & Yang, F. Gelatin-based microribbon hydrogels accelerate cartilage formation by mesenchymal stem cells in three dimensions. Tissue Eng. A 24, 1631–1640 (2018).
Lee, H., Gu, L., Mooney, D. J., Levenston, M. E. & Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 16, 1243–1251 (2017).
Richardson, B. M., Wilcox, D. G., Randolph, M. A. & Anseth, K. S. Hydrazone covalent adaptable networks modulate extracellular matrix deposition for cartilage tissue engineering. Acta Biomater. 83, 71–82 (2019).
Gong, Z. et al. Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proc. Natl Acad. Sci. USA 115, E2686–E2695 (2018).
Charrier, E. E., Pogoda, K., Wells, R. G. & Janmey, P. A. Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat. Commun. 9, 449 (2018).
McKinnon, D. D., Domaille, D. W., Cha, J. N. & Anseth, K. S. Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Adv. Mater. 26, 865–872 (2014).
Cameron, A. R., Frith, J. E. & Cooper-White, J. J. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32, 5979–5993 (2011).
Chaudhuri, O. et al. Substrate stress relaxation regulates cell spreading. Nat. Commun. 6, 6364 (2015).
Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016).
Lee, H., Stowers, R. & Chaudhuri, O. Volume expansion and TRPV4 activation regulate stem cell fate in three-dimensional microenvironments. Nat. Commun. 10, 529 (2019).
Wisdom, K. M. et al. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 9, 4144 (2018).
Lee, J. et al. Early induction of a prechondrogenic population allows efficient generation of stable chondrocytes from human induced pluripotent stem cells. FASEB J. 29, 3399–3410 (2015).
Darling, E. M., Wilusz, R. E., Bolognesi, M. P., Zauscher, S. & Guilak, F. Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy. Biophys. J. 98, 2848–2856 (2010).
Alexopoulos, L. G., Williams, G. M., Upton, M. L., Setton, L. A. & Guilak, F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J. Biomech. 38, 509–517 (2005).
McLeod, M. A., Wilusz, R. E. & Guilak, F. Depth-dependent anisotropy of the micromechanical properties of the extracellular and pericellular matrices of articular cartilage evaluated via atomic force microscopy. J. Biomech. 46, 586–592 (2013).
Chen, D. et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 5, 16044 (2017).
Wenham, C. Y. J. & Conaghan, P. G. The role of synovitis in osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2, 349–359 (2010).
Raghu, H. et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 76, 914–922 (2017).
Zhao, X. Y. et al. CCL3 serves as a potential plasma biomarker in knee degeneration (osteoarthritis). Osteoarthr. Cartil. 23, 1405–1411 (2015).
Yan, D. et al. Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes. Arthritis Res. Ther. 13, R130 (2011).
Scanzello, C. R. Chemokines and inflammation in osteoarthritis: insights from patients and animal models. J. Orthop. Res. 35, 735–739 (2017).
Leah, E. Experimental arthritis: GM-CSF mediates pain and disease in a mouse model of osteoarthritis. Nat. Rev. Rheumatol. 8, 634 (2012).
Cook, A. D. et al. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis Res. Ther. 14, R199 (2012).
Erickson, G. R., Alexopoulos, L. G. & Guilak, F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J. Biomech. 34, 1527–1535 (2001).
Loeser, R. F., Erickson, E. A. & Long, D. L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 20, 581–586 (2008).
Saklatvala, J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr. Drug Targets 8, 305–313 (2007).
Thalhamer, T., McGrath, M. A. & Harnett, M. M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 47, 409–414 (2007).
Ge, H., Zou, F., Li, Y., Liu, A. & Tu, M. JNK pathway in osteoarthritis: pathological and therapeutic aspects. J. Recept. Signal Transduct. 37, 431–436 (2017).
Melas, I. N. et al. Modeling of signaling pathways in chondrocytes based on phosphoproteomic and cytokine release data. Osteoarthr. Cartil. 22, 509–518 (2014).
Corr, M. Wnt–β-catenin signaling in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 4, 550–556 (2008).
Miclea, R. L. et al. Inhibition of Gsk3β in cartilage induces osteoarthritic features through activation of the canonical Wnt signaling pathway. Osteoarthr. Cartil. 19, 1363–1372 (2011).
Guidotti, S. et al. GSK3β inactivation affects chondrocyte mitochondria leading to oxidative DNA damage, GADD45 beta induction, hypertrophy and cellular senescence. Osteoarthr. Cartil. 22, S163–S164 (2014).
O’Conor, C. J., Leddy, H. A., Benefield, H. C., Liedtke, W. B. & Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl Acad. Sci. USA 111, 1316–1321 (2014).
O’Conor, C. J. et al. Cartilage-specific knockout of the mechanosensory ion channel TRPV4 decreases age-related osteoarthritis. Sci. Rep. 6, 29053 (2016).
Jin, M. et al. Determinants of TRPV4 activity following selective activation by small molecule agonist GSK1016790A. PLoS ONE 6, e0016713 (2011).
Ramos, Y. F. M. et al. Genes involved in the osteoarthritis process identified through genome wide expression analysis in articular cartilage; the RAAK study. PLoS ONE 9, e103056 (2014).
Askari, A. et al. Increased serum levels of IL-17A and IL-23 are associated with decreased vitamin D3 and increased pain in osteoarthritis. PLoS ONE 11, e0164757 (2016).
Taylor, S. E. B. et al. Identification of human juvenile chondrocyte-specific factors that stimulate stem cell growth. Tissue Eng. A 22, 645–653 (2016).
Aigner, T., Zien, A., Gehrsitz, A., Gebhard, P. M. & McKenna, L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 44, 2777–2789 (2001).
Guo, M. et al. Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc. Natl Acad. Sci. USA 114, E8618–E8627 (2017).
Stroka, K. M. et al. Water permeation drives tumor cell migration in confined microenvironments. Cell 157, 611–623 (2014).
Zlotek-Zlotkiewicz, E., Monnier, S., Cappello, G., Berre, M. L. & Piel, M. Optical volume and mass measurements show that mammalian cells swell during mitosis. J. Cell Biol. 211, 765–774 (2015).
Bader, D. L., Salter, D. M. & Chowdhury, T. T. Biomechanical influence of cartilage homeostasis in health and disease. Arthritis 2011, 979032 (2011).
Wu, Q. et al. Smurf2 induces degradation of GSK-3β and upregulates β-catenin in chondrocytes: a potential mechanism for Smurf2-induced degeneration of articular cartilage. Exp. Cell Res. 315, 2386–2398 (2009).
Boyle, W. J. et al. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell 64, 573–584 (1991).
Lewis, R., Feetham, C. H. & Barrett-Jolley, R. Cell volume regulation in chondrocytes. Cell. Physiol. Biochem. 28, 1111–1122 (2011).
Servin-Vences, M. Rocio, Moroni, M., Lewin, G. & Poole, K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. eLife 6, e21074 (2017).
O’Connor, C. J. Increased susceptibility of Trpv4-deficient mice to obesity and obesity-induced osteoarthritis with very high-fat diet. Ann. Rheum. Dis. 72, 300–4 (2013).
van der Eerden, B. C. TRPV4 deficiency causes sexual dimorphism in bone metabolism and osteoporotic fracture risk. Bone 57, 443–54 (2013).
Clark, A. L., Votta, B. J., Kumar, S., Liedtke, W. & Guilak, F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 62, 2973–2983 (2010).
McNulty, A. L., Leddy, H. A., Liedtke, W. & Guilak, F. TRPV4 as a therapeutic target for joint diseases. Naunyn. Schmiedebergs Arch. Pharmacol. 388, 437–450 (2015).
Jones, R. C. et al. Piezo1 expression is increased in response to non-invasive impact of mouse knee joint. Osteoarthr. Cartil. 26, S113–S114 (2018).
Lee, W. et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl Acad. Sci. USA 111, E5114–E5122 (2014).
Pritchard, S. & Guilak, F. Effects of interleukin-1 on calcium signaling and the increase of filamentous actin in isolated and in situ articular chondrocytes. Arthritis Rheum. 54, 2164–2174 (2006).
Beekman, B., Verzijl, N., de Roos, J. A. D. M. & TeKoppele, J. M. Matrix degradation by chondrocytes cultured in alginate: IL-1β induces proteoglycan degradation and proMMP synthesis but does not result in collagen degradation. Osteoarthr. Cartil. 6, 330–340 (1998).
Walczysko, P., Wagner, E. & Albrechtová, J. T. P. Use of co-loaded Fluo-3 and Fura red fluorescent indicators for studying the cytosolic Ca2+ concentrations distribution in living plant tissue. Cell Calcium 28, 23–32 (2000).
Agarwal, P. et al. Dataset for ‘A dysfunctional TRPV4–GSK3β pathway prevents osteoarthritic chondrocytes from sensing changes in extracellular matrix viscoelasticity’. figshare https://doi.org/10.6084/m9.figshare.13184909 (2021).
Acknowledgements
We thank Y. Rosenberg-Hasson at the Stanford Human Immune Profiling Center for help with the Luminex analysis. These studies were supported by funding from the Stanford Bio-X Interdisciplinary Initiatives Seed Grants Program (IIP) (R9-52 to N.B. and O.C.), National Institutes of Health grants (R01 AR070864 and R01 AR070865 to N.B. and R21 AR074070 to O.C.) and a Stanford Bio-X fellowship (to H.-p.L.).
Author information
Authors and Affiliations
Contributions
P.A., H.-p.L., O.C. and N.B. designed the overall study. P.A. and H.-p.L. conducted experiments and analysed the data. P.S. and F.G. contributed to discussions and data interpretation. S.G. provided samples from human patients with OA. P.A., H.-p.L., O.C. and N.B. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures.
Rights and permissions
About this article
Cite this article
Agarwal, P., Lee, Hp., Smeriglio, P. et al. A dysfunctional TRPV4–GSK3β pathway prevents osteoarthritic chondrocytes from sensing changes in extracellular matrix viscoelasticity. Nat Biomed Eng 5, 1472–1484 (2021). https://doi.org/10.1038/s41551-021-00691-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00691-3
This article is cited by
-
LIPUS regulates the progression of knee osteoarthritis in mice through primary cilia-mediated TRPV4 channels
Apoptosis (2024)
-
Cell–extracellular matrix mechanotransduction in 3D
Nature Reviews Molecular Cell Biology (2023)
-
Mechanotransduction pathways in articular chondrocytes and the emerging role of estrogen receptor-α
Bone Research (2023)
-
Tailorable non-linear viscoelastic behavior of hydrogels
Mechanics of Time-Dependent Materials (2023)
-
Senescence in osteoarthritis: from mechanism to potential treatment
Arthritis Research & Therapy (2022)