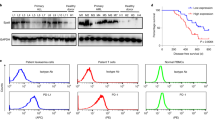
Abstract
The formulations of peptide-based antitumour vaccines being tested in clinical studies are generally associated with weak potency. Here, we show that pharmacokinetically tuning the responses of peptide vaccines by fusing the peptide epitopes to carrier proteins optimizes vaccine immunogenicity in mice. In particular, we show in immunized mice that the carrier protein transthyretin simultaneously optimizes three factors: efficient antigen uptake in draining lymphatics from the site of injection, protection of antigen payloads from proteolytic degradation and reduction of antigen presentation in uninflamed distal lymphoid organs. Optimizing these factors increases vaccine immunogenicity by up to 90-fold and maximizes the responses to viral antigens, tumour-associated antigens, oncofetal antigens and shared neoantigens. Protein–peptide epitope fusions represent a facile and generalizable strategy for enhancing the T-cell responses elicited by subunit vaccines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the paper and its Supplementary information. The associated raw data are too numerous to be readily shared publicly but can be made available from the corresponding author on reasonable request.
References
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Kantarjian, H. et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 376, 836–847 (2017).
Andtbacka, R. H. I. et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33, 2780–2788 (2015).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Rizvi, N. A. et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Hodi, F. S. et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc. Natl Acad. Sci. USA 105, 3005–3010 (2008).
Ott, P. A. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017).
Keskin, D. B. et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 565, 234–239 (2019).
Liu, H. et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522 (2014).
McLennan, D. N., Porter, C. J. H. & Charman, S. A. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov. Today Technol. 2, 89–96 (2005).
Brinckerhoff, L. H. et al. Terminal modifications inhibit proteolytic degradation of an immunogenic mart-127–35 peptide: implications for peptide vaccines. Int. J. Cancer 83, 326–334 (1999).
Moynihan, K. D. et al. Enhancement of peptide vaccine immunogenicity by increasing lymphatic drainage and boosting serum stability. Cancer Immunol. Res. 6, 1025–1038 (2018).
Moon, J. J. et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 10, 243–251 (2011).
Kuai, R., Ochyl, L. J., Bahjat, K. S., Schwendeman, A. & Moon, J. J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 16, 489–496 (2017).
Nembrini, C. et al. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proc. Natl Acad. Sci. USA 108, E989–E997 (2011).
Bonifaz, L. C. et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 199, 815–824 (2004).
Kretz-Rommel, A. et al. In vivo targeting of antigens to human dendritic cells through DC-SIGN elicits stimulatory immune responses and inhibits tumor growth in grafted mouse models. J. Immunother. 30, 715–726 (2007).
Johansen, P. et al. Direct intralymphatic injection of peptide vaccines enhances immunogenicity. Eur. J. Immunol. 35, 568–574 (2005).
Jewell, C. M., Bustamante Lopez, S. C. & Irvine, D. J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl Acad. Sci. USA 108, 15745–15750 (2011).
Supersaxo, A., Hein, W. R. & Steffen, H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm. Res. 7, 167–169 (1990).
Feltkamp, M. C. W. et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur. J. Immunol. 23, 2242–2249 (1993).
Hailemichael, Y. et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat. Med. 19, 465–472 (2013).
Burdette, D. L. et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478, 515–518 (2011).
Guevara-Patiño, J. A. et al. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J. Clin. Invest. 116, 1382–1390 (2006).
van Stipdonk, M. J. B. et al. Design of agonistic altered peptides for the robust induction of CTL directed towards H-2Db in complex with the melanoma-associated epitope gp100. Cancer Res. 69, 7784–7792 (2009).
Mennuni, C. et al. Efficient induction of T-cell responses to carcinoembryonic antigen by a heterologous prime-boost regimen using DNA and adenovirus vectors carrying a codon usage optimized cDNA. Int. J. Cancer 117, 444–455 (2005).
Han, S., Asoyan, A., Rabenstein, H., Nakano, N. & Obst, R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc. Natl Acad. Sci. USA 107, 20453–20458 (2010).
Ramsdell, F. & Fowlkes, B. J. Maintenance of in vivo tolerance by persistence of antigen. Science 257, 1130–1134 (1992).
Ehl, S. et al. Antigen persistence and time of T-cell tolerization determine the efficacy of tolerization protocols for prevention of skin graft rejection. Nat. Med. 4, 1015–1019 (1998).
Warren, K. G., Catz, I., Ferenczi, L. Z. & Krantz, M. J. Intravenous synthetic peptide MBP8298 delayed disease progression in an HLA class II-defined cohort of patients with progressive multiple sclerosis: results of a 24-month double-blind placebo-controlled clinical trial and 5 years of follow-up treatment. Eur. J. Neurol. 13, 887–895 (2006).
Bielekova, B. et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 6, 1167–1175 (2000).
Hanson, M. C. et al. Nanoparticulate STING agonists are potent lymph node–targeted vaccine adjuvants. J. Clin. Invest. 125, 2532–2546 (2015).
Churlaud, G. et al. Human and mouse CD8+CD25+FOXP3+ regulatory T cells at steady state and during interleukin-2 therapy. Front. Immunol. 6, 171 (2015).
Yu, Y. et al. Recent advances in CD8+ regulatory T cell research. Oncol. Lett. 15, 8137–8194 (2018).
Tsai, S., Clemente-Casares, X. & Santamaria, P. CD8+ Tregs in autoimmunity: learning ‘self’-control from experience. Cell. Mol. Life Sci. 68, 3781–3795 (2011).
Ingenbleek, Y. & Young, V. Transthyretin (prealbumin) in health and disease: nutritional implications. Annu. Rev. Nutr. 14, 495–533 (1994).
Terje Andersen, J., Bekele Daba, M., Berntzen, G., Michaelsen, T. E. & Sandlie, I. Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. J. Biol. Chem. 285, 4826–4836 (2010).
McCutchen, S. L., Colon, W. & Kelly, J. W. Transthyretin mutation Leu-55-Pro significantly alters tetramer stability and increases amyloidogenicity. Biochemistry 32, 12119–12127 (1993).
Prior, I. A., Lewis, P. D. & Mattos, C. A comprehensive survey of ras mutations in cancer. Cancer Res. 72, 2457–2467 (2012).
Waters, A. M. & Der, C. J. KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb. Perspect. Med. 8, a031435 (2018).
Bender, S. et al. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell 24, 660–672 (2013).
Schwartzentruber, J. et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482, 226–231 (2012).
Ochs, K. et al. K27M-mutant histone-3 as a novel target for glioma immunotherapy. Oncoimmunology 6, e1328340 (2017).
Wang, Q. J. et al. Identification of T-cell receptors targeting KRAS-mutated human tumors. Cancer Immunol. Res. 4, 204–214 (2016).
Chheda, Z. S. et al. Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J. Exp. Med. 215, 141–157 (2018).
Zom, G. G. et al. Efficient induction of antitumor immunity by synthetic Toll-like receptor ligand–peptide conjugates. Cancer Immunol. Res. 2, 756–764 (2014).
Qiu, F. et al. Poly(propylacrylic acid)-peptide nanoplexes as a platform for enhancing the immunogenicity of neoantigen cancer vaccines. Biomaterials 182, 82–91 (2018).
Deng, L. et al. Heterosubtypic influenza protection elicited by double-layered polypeptide nanoparticles in mice. Proc. Natl Acad. Sci. USA 115, E7758–E7767 (2018).
Harris, J. R. & Markl, J. Keyhole limpet hemocyanin (KLH): a biomedical review. Micron 30, 597–623 (1999).
Kim, S. K. et al. Comparison of the effect of different immunological adjuvants on the antibody and T-cell response to immunization with MUC1-KLH and GD3-KLH conjugate cancer vaccines. Vaccine 18, 597–603 (1999).
Lynn, G. M. et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 33, 1201–1210 (2015).
Wu, T. Y.-H. et al. Rational design of small molecules as vaccine adjuvants. Sci. Transl. Med. 6, 263ra160 (2014).
Duperret, E. K. et al. A synthetic DNA, multi-neoantigen vaccine drives predominately MHC class I CD8+ T-cell responses, impacting tumor challenge. Cancer Immunol. Res. 7, 174–182 (2019).
Walters, J. N. et al. A novel DNA vaccine platform enhances neo-antigen-like T-cell responses against WT1 to break tolerance and induce anti-tumor immunity. Mol. Ther. 25, 976–988 (2017).
Geall, A. J. et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl Acad. Sci. USA 109, 14604–14609 (2012).
Horton, H. M. et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 68, 8049–8057 (2008).
Nomura, L. E., Walker, J. M. & Maecker, H. T. Optimization of whole blood antigen-specific cytokine assays for CD4+ T cells. Cytometry 40, 60–68 (2000).
Chen, T. F., de Picciotto, S., Hackel, B. J. & Wittrup, K. D. Engineering Fibronectin-Based binding proteins by yeast surface display. Methods Enzymol. 523, 303–326 (2013).
Acknowledgements
We thank the Koch Institute Swanson Biotechnology Center, particularly the Flow Cytometry and Animal Imaging and Preclinical Testing Core Facilities, as well as the Biophysical Instrumentation Facility for technical support. This work was supported, in part, by the National Institutes of Health (grant no. CA174795 and the National Institute of General Medical Sciences-NIH Interdepartmental Biotechnology Training Program). D.J.I. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
N.K.M., K.D.W. and D.J.I. designed the studies and wrote the manuscript. N.K.M., R.V.P. and A.P.S. carried out experiments. K.D.M., A.M.R., N.M. and K.R. assisted with experiments. J.M.-F. provided the DEC-205 binders. S.N.B. lent expertise to the tolerization studies.
Corresponding authors
Ethics declarations
Competing interests
D.J.I., K.D.W., N.K.M. and K.R. are inventors on a related patent that covers the primary technology outlined in the manuscript. Massachusetts Institute of Technology is the assignee for this patent application. Application number: US15/452,266. D.J.I. has ownership interest in and is a consultant/advisory board member for Elicio Therapeutics. The remaining authors report no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, tables and list of abbreviations.
Rights and permissions
About this article
Cite this article
Mehta, N.K., Pradhan, R.V., Soleimany, A.P. et al. Pharmacokinetic tuning of protein–antigen fusions enhances the immunogenicity of T-cell vaccines. Nat Biomed Eng 4, 636–648 (2020). https://doi.org/10.1038/s41551-020-0563-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-0563-4
This article is cited by
-
Targeted modulation of immune cells and tissues using engineered biomaterials
Nature Reviews Bioengineering (2023)
-
Maximizing response to intratumoral immunotherapy in mice by tuning local retention
Nature Communications (2022)
-
Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment
Nature Communications (2022)
-
Targeting public neoantigens for cancer immunotherapy
Nature Cancer (2021)
-
Designing spatial and temporal control of vaccine responses
Nature Reviews Materials (2021)