Abstract
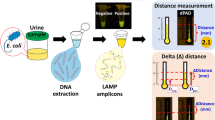
The point-of-care detection of pathogens in biological samples in resource-limited settings should be inexpensive, rapid, portable, simple and accurate. Here, we describe a custom-made fidget spinner that rapidly concentrates pathogens in 1-ml samples of undiluted urine by more than 100-fold for the on-device colorimetric detection of bacterial load and pathogen identification. In Tiruchirappalli, India, the device enabled the on-site detection of infection with the naked eye within 50 min in urine samples from 39 patients suspected of having a urinary tract infection. We also show that, in 30 clinical samples of urinary tract infection, the device can be used to perform an antimicrobial susceptibility test for the antimicrobial drugs ciprofloxacin and cefazolin within 120 min. The fidget spinner could be used in low-resource settings as an inexpensive handheld point-of-care device for the rapid concentration and detection of pathogens in urine samples.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the findings in this study are available within the paper and its Supplementary information. The raw and analysed datasets are too numerous to be readily shared publicly but are available for research purposes from the corresponding author on reasonable request.
Code availability
The custom Matlab code for data analysis is provided at https://github.com/yoonkyoungcho/Fidget.
References
Vashist, S. K., Luppa, P. B., Yeo, L. Y., Ozcan, A. & Luong, J. H. T. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol. 33, 692–705 (2015).
Pollock, N. R. et al. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci. Transl. Med. 4, 152ra129 (2012).
Ng, A. H. C. et al. A digital microfluidic system for serological immunoassays in remote settings. Sci. Transl. Med. 10, eaar6076 (2018).
Watkins, N. N. et al. Microfluidic CD4+ and CD8+ T lymphocyte counters for point-of-care HIV diagnostics using whole blood. Sci. Transl. Med. 5, 214ra170 (2013).
Manz, A., Graber, N. & Widmer, H. M. Miniaturized total chemical analysis systems: a novel concept for chemical sensing. Sens. Actuators B 1, 244–248 (1990).
deMello, A. J. Control and detection of chemical reactions in microfluidic systems. Nature 442, 394–402 (2006).
Whitesides, G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006).
Mauk, M. G. Calling in the test: smartphone-based urinary sepsis diagnostics. EBioMedicine 37, 11–12 (2018).
Barnes, L. et al. Smartphone-based pathogen diagnosis in urinary sepsis patients. EBioMedicine 36, 73–82 (2018).
Laksanasopin, T. et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci. Transl. Med. 7, 273re1 (2015).
S. T. Thomas, C. Heneghan, C. P. Price, A. V. d. Bruel, A. Plüddemann. Point-of-Care Testing for Urinary Tract Infections. Horizon Scan Report 0045 (National Institute for Health and Research, 2016).
Yager, P., Domingo, G. J. & Gerdes, J. Point-of-care diagnostics for global health. Annu. Rev. Biomed. Eng. 10, 107–144 (2008).
Michael, I., Kim, T.-H., Sunkara, V. & Cho, Y.-K. Challenges and opportunities of centrifugal microfluidics for extreme point-of-care testing. Micromachines 7, 32 (2016).
Posthuma-Trumpie, G. A., Korf, J. & van Amerongen, A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. a literature survey. Anal. Bioanal. Chem. 393, 569–582 (2009).
Davenport, M. et al. New and developing diagnostic technologies for urinary tract infections. Nat. Rev. Urol. 14, 298–310 (2017).
Urinary Tract Infections in Infants and Children in Developing Countries in the Context of IMCI. 1–8 (WHO, 2005).
Hooton, T. M. Uncomplicated urinary tract infection. N. Engl. J. Med. 366, 1028–1037 (2012).
Wilson, M. L. & Gaido, L. Laboratory diagnosis of urinary tract infections in adult patients. Clin. Infect. Dis. 38, 1150–1158 (2004).
Gilbert, N. M. et al. Urinary tract infection as a preventable cause of pregnancy complications: opportunities, challenges, and a global call to action. Glob. Adv. Health Med. 2, 59–69 (2013).
Schmiemann, G., Kniehl, E., Gebhardt, K., Matejczyk, M. M. & Hummers-Pradier, E. The diagnosis of urinary tract infection. Dtsch. Aerztebl. Int. 107, 361–367 (2010).
Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 7, 653–660 (2010).
Schecter, R. A., Shah, J., Fruitman, K. & Milanaik, R. L. Fidget spinners: purported benefits, adverse effects and accepted alternatives. Curr. Opin. Pediatr. 29, 616–618 (2017).
Bhamla, M. S. et al. Hand-powered ultralow-cost paper centrifuge. Nat. Biomed. Eng. 1, 0009 (2017).
Zhang, L. et al. Hand-powered centrifugal microfluidic platforms inspired by a spinning top for sample-to-answer diagnostics of nucleic acids. Lab Chip 18, 610–619 (2018).
Liu, C. H. et al. Blood plasma separation using a fidget-spinner. Anal. Chem. 91, 1247–1253 (2019).
Sackmann, E. K., Fulton, A. L. & Beebe, D. J. The present and future role of microfluidics in biomedical research. Nature 507, 181–189 (2014).
Gorkin, R. et al. Centrifugal microfluidics for biomedical applications. Lab Chip 10, 1758–1773 (2010).
Kim, T.-H. et al. FAST: size-selective, clog-free isolation of rare cancer cells from whole blood at a liquid-liquid interface. Anal. Chem. 89, 1155–1162 (2017).
Tsukatani, T. et al. Comparison of the WST-8 colorimetric method and the CLSI broth microdilution method for susceptibility testing against drug-resistant bacteria. J. Microbiol. Methods 90, 160–166 (2012).
Aguiar, J. P. Evaluation of empirical antibiotic therapy for the treatment of community-acquired urinary tract infections (CA-UTI). Int. Arch. Clin. Pharmacol. 1, 002 (2017).
National Treatment Guidelines for Antimicrobial Use in Infectious Diseases, India 1–64 (National Centre For Disease Control, 2008).
Mishra, B., Srivastava, S., Singh, K., Pandey, A. & Agarwal, J. Symptom-based diagnosis of urinary tract infection in women: are we over-prescribing antibiotics? Int. J. Clin. Pract. 66, 493–498 (2012).
Hasan, A. S. K., Kumar, N. T., Kishan, R. N. & Neetha, K. Laboratory diagnosis of urinary tract infections using diagnostics tests in adult patients. Int. J. Res. Med. Sci. 2, 415–421 (2014).
George, C. E., Norman, G., Ramana, G. V., Mukherjee, D. & Rao, T. Treatment of uncomplicated symptomatic urinary tract infections: resistance patterns and misuse of antibiotics. J. Fam. Med. Prim. Care 4, 416–421 (2015).
European Confederation of Laboratory Medicine. European urinalysis guidelines. Scand. J. Clin. Lab. Invest. Suppl. 231, 1–86 (2000)..
Chokshi, M. et al. Health systems in India. J. Perinatol. 36, S9–S12 (2016).
Guidelines for Good Clinical Laboratory Practices (GCLP) (Indian Council of Medical Research, 2008).
Kuper, K. M., Boles, D. M., Mohr, J. F. & Wanger, A. Antimicrobial susceptibility testing: a primer for clinicians. Pharmacotherapy 29, 1326–1343 (2009).
Avesar, J. et al. Rapid phenotypic antimicrobial susceptibility testing using nanoliter arrays. Proc. Natl Acad. Sci. USA 114, E5787–E5795 (2017).
Amábile-Cuevas, C. F. Basis for a cheap method for detecting bacteria and assessing their antibiotic susceptibility in urine samples. J. Glob. Antimicrob. Resist. 1, 17–21 (2013).
Kettler, H., White, K. & Hawkes, S. Mapping the Landscape of Diagnostics for Sexually Transmitted Infections (UNICEF/UNDP/World Bank/WHO, 2004).
Lee, A. et al. All-in-one centrifugal microfluidic device for size-selective circulating tumor cell isolation with high purity. Anal. Chem. 86, 11349–11356 (2014).
Lee, B. S. et al. Fully integrated lab-on-a-disc for simultaneous analysis of biochemistry and immunoassay from whole blood. Lab Chip 11, 70–78 (2011).
Yaguchi, T. et al. Aqueous two-phase system-derived biofilms for bacterial interaction studies. Biomacromolecules 13, 2655–2661 (2012).
Hellens, R. P., Edwards, E. A., Leyland, N. R., Bean, S. & Mullineaux, P. M. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42, 819–832 (2000).
Olivo-Marin, J.-C. Extraction of spots in biological images using multiscale products. Pattern Recognit. 35, 1989–1996 (2002).
Photography–Electronic Still Picture Imaging–Resolution and Spatial Frequency Responses 3rd edn (International Organization for Standardization, 2017).
International Color Consortium Specification ICC.1:2004–10 (ICC, 2006).
Acknowledgements
We thank S. Manivanan for his support in the clinical sample testing. We thank J. Oh for discussions about the modelling. This work is supported by a grant from the Institute for Basic Science of Korea (grant no. IBS-R020-D1) and the Korean Health Technology R&D Project of the Ministry of Health and Welfare (grant no. HI12C1845).
Author information
Authors and Affiliations
Contributions
I.M., D.K. and Y.-K.C. conceived and designed the research. I.M., D.K., O.G., Sumit K., J.C., Saravana K., D.Y.K., J.P., H.Y.J. and T.S.K. performed the research. I.M., D.K., O.G., H.Y.J., S.Kwon and Y.-K.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
I.M., D.K., D.Y.K. and Y.-K.C. are inventors of a patent (10-2103784, Korea). Y.-K.C. is an inventor of a filed patent (14/780,002, USA). All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary notes, figures, tables, references and video captions.
Supplementary Video 1
Commercial fidget spinner (left) and Dx-FS (right), operated by a user.
Supplementary Video 2
Urine test procedure using Dx-FS.
Rights and permissions
About this article
Cite this article
Michael, I., Kim, D., Gulenko, O. et al. A fidget spinner for the point-of-care diagnosis of urinary tract infection. Nat Biomed Eng 4, 591–600 (2020). https://doi.org/10.1038/s41551-020-0557-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-0557-2
This article is cited by
-
An ultralow-cost portable centrifuge from discarded materials for medical applications
Scientific Reports (2023)
-
Smartphone-based platforms implementing microfluidic detection with image-based artificial intelligence
Nature Communications (2023)
-
Materials, assemblies and reaction systems under rotation
Nature Reviews Materials (2022)
-
A film-lever actuated switch technology for multifunctional, on-demand, and robust manipulation of liquids
Nature Communications (2022)
-
Multiplexed paper-based assay for personalized antimicrobial susceptibility profiling of Carbapenem-resistant Enterobacterales performed in a rechargeable coffee mug
Scientific Reports (2022)