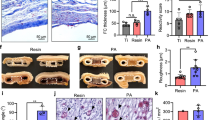
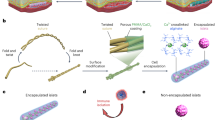
Abstract
The long-term function of transplanted therapeutic cells typically requires systemic immune suppression. Here, we show that a retrievable implant comprising a silicone reservoir and a porous polymeric membrane protects human cells encapsulated in it after implant transplantation in the intraperitoneal space of immunocompetent mice. Membranes with pores 1 µm in diameter allowed host macrophages to migrate into the device without the loss of transplanted cells, whereas membranes with pore sizes <0.8 µm prevented their infiltration by immune cells. A synthetic polymer coating prevented fibrosis and was necessary for the long-term function of the device. For >130 days, the device supported human cells engineered to secrete erythropoietin in immunocompetent mice, as well as transgenic human cells carrying an inducible gene circuit for the on-demand secretion of erythropoietin. Pancreatic islets from rats encapsulated in the device and implanted in diabetic mice restored normoglycaemia in the mice for over 75 days. The biocompatible device provides a retrievable solution for the transplantation of engineered cells in the absence of immunosuppression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the findings in this study are available within the paper and its Supplementary Information. The raw and analysed datasets are too numerous to be readily shared publicly. Source data for the figures are available for research purposes from the corresponding author on reasonable request.
References
Towards advanced cell therapies. Nat. Biomed. Eng. 2, 339–340 (2018).
Sedlmayer, F., Aubel, D. & Fussenegger, M. Synthetic gene circuits for the detection, elimination and prevention of disease. Nat. Biomed. Eng. 2, 399–415 (2018).
Aijaz, A. et al. Biomanufacturing for clinically advanced cell therapies. Nat. Biomed. Eng. 2, 362–376 (2018).
Xie, M. et al. β-cell-mimetic designer cells provide closed-loop glycemic control. Science 354, 1296–1301 (2016).
Kojima, R. et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 9, 5066 (2018).
Power, A. T. et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol. Ther. 15, 123–130 (2007).
Chassin, H. et al. Sensing and responding to allergic response cytokines through a genetically encoded circuit. Nat. Commun. 8, 1101 (2017).
Pagliuca, F. W. et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014).
Millman, J. R. et al. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat. Commun. 7, 11463 (2016).
Desai, T. & Shea, L. D. Advances in islet encapsulation technologies. Nat. Rev. Drug Discov. 16, 338–350 (2017).
Wood, K. J., Issa, F. & Hester, J. Understanding stem cell immunogenicity in therapeutic applications. Trends Immunol. 37, 5–16 (2016).
Phelps, E. A. et al. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv. Mater. 24, 64–70 (2012).
An, D. et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc. Natl Acad. Sci. USA 115, E263–E272 (2018).
An, D. et al. Developing robust, hydrogel-based, nanofiber-enabled encapsulation devices (NEEDs) for cell therapies. Biomaterials 37, 40–48 (2015).
Weir, G. C. Islet encapsulation: advances and obstacles. Diabetologia 56, 1458–1461 (2013).
Hwa, A. J. & Weir, G. C. Transplantation of macroencapsulated insulin-producing cells. Curr. Diab. Rep. 18, 50 (2018).
Colton, C. K. in Principles of Tissue Engineering 543–562 (Academic Press, 2014).
Nyitray, C. E. et al. Polycaprolactone thin-film micro- and nanoporous cell-encapsulation devices. ACS Nano 9, 5675–5682 (2015).
Chang, R. et al. Nanoporous immunoprotective device for stem-cell-derived β-cell replacement therapy. ACS Nano 11, 7747–7757 (2017).
Kumagai-Braesch, M. et al. The TheraCyte device protects against islet allograft rejection in immunized hosts. Cell Transplant. 22, 1137–1146 (2013).
Pedraza, E., Coronel, M. M., Fraker, C. A., Ricordi, C. & Stabler, C. L. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc. Natl Acad. Sci. USA 109, 4245–4250 (2012).
Paul, C. D., Mistriotis, P. & Konstantopoulos, K. Cancer cell motility: lessons from migration in confined spaces. Nat. Rev. Cancer 17, 131–140 (2017).
Anderson, J. M., Rodriguez, A. & Chang, D. T. Foreign body reaction to biomaterials. Semin. Immunol. 20, 86–100 (2008).
Vegas, A. J. et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol. 34, 345–352 (2016).
Vegas, A. J. et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived β-cells in immune-competent mice. Nat. Med. 22, 306–311 (2016).
Bochenek, M. A. et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat. Biomed. Eng. 2, 810–821 (2018).
Pullen, L. C. Stem cell-derived pancreatic progenitor cells have now been transplanted into patients: report from IPITA 2018. Am. J. Transplant. 18, 1581–1582 (2018).
Mahou, R., Zhang, D. K. Y., Vlahos, A. E. & Sefton, M. V. Injectable and inherently vascularizing semi-interpenetrating polymer network for delivering cells to the subcutaneous space. Biomaterials 131, 27–35 (2017).
Weaver, J. D. et al. Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials 172, 54–65 (2018).
Ludwig, B. et al. Transplantation of human islets without immunosuppression. Proc. Natl Acad. Sci. USA 110, 19054–19058 (2013).
Whyte, W. et al. Sustained release of targeted cardiac therapy with a replenishable implanted epicardial reservoir. Nat. Biomed. Eng. 2, 416–428 (2018).
Lee, K. S. & Ram, R. J. Plastic–PDMS bonding for high pressure hydrolytically stable active microfluidics. Lab Chip 9, 1618–1624 (2009).
Lovett, M., Lee, K., Edwards, A. & Kaplan, D. L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 15, 353–370 (2009).
Mendelsohn, A. & Desai, T. Inorganic nanoporous membranes for immunoisolated cell-based drug delivery. Adv. Exp. Med. Biol. 670, 104–125 (2010).
Scalea, J., Hanecamp, I., Robson, S. C. & Yamada, K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation 19, 23–30 (2012).
Leader, B., Baca, Q. J. & Golan, D. E. Protein therapeutics: a summary and pharmacological classification. Nat. Rev. Drug Discov. 7, 21–39 (2008).
Lee, D. E., Son, W., Ha, B. J., Oh, M. S. & Yoo, O. J. The prolonged half-lives of new erythropoietin derivatives via peptide addition. Biochem. Biophys. Res. Commun. 339, 380–385 (2006).
Régulier, E., Schneider, B. L., Déglon, N., Beuzard, Y. & Aebischer, P. Continuous delivery of human and mouse erythropoietin in mice by genetically engineered polymer encapsulated myoblasts. Gene Ther. 5, 1014–1022 (1998).
Schweicher, J., Nyitray, C. & Desai, T. A. Membranes to achieve immunoprotection of transplanted islets. Front. Biosci. 19, 49–76 (2014).
Chong, A. S., Alegre, M.-L., Miller, M. L. & Fairchild, R. L. Lessons and limits of mouse models. Cold Spring Harb. Perspect. Med. 3, a015495 (2013).
Tucker, S. P., Melsen, L. R. & Compans, R. W. Migration of polarized epithelial cells through permeable membrane substrates of defined pore size. Eur. J. Cell Biol. 58, 280–290 (1992).
Bryers, J. D., Giachelli, C. M. & Ratner, B. D. Engineering biomaterials to integrate and heal: the biocompatibility paradigm shifts. Biotechnol. Bioeng. 109, 1898–1911 (2012).
Whyte, W. et al. Sustained release of targeted cardiac therapy with a replenishable implanted epicardial reservoir. Nat. Biomed. Eng. 2, 416–428 (2018).
Zhang, L. et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 31, 553–556 (2013).
Smith, R. S. et al. Vascular catheters with a nonleaching poly-sulfobetaine surface modification reduce thrombus formation and microbial attachment. Sci. Transl. Med. 4, 153ra132 (2012).
Xie, X. et al. Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer. Nat. Biomed. Eng. 2, 894–906 (2018).
Jin, X., Yuan, J. & Shen, J. Zwitterionic polymer brushes via dopamine-initiated ATRP from PET sheets for improving hemocompatible and antifouling properties. Colloids Surf. B Biointerfaces 145, 275–284 (2016).
Veiseh, O. et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 14, 643–651 (2015).
Tireli, M. et al. Solvent-free copper-catalyzed click chemistry for the synthesis of N-heterocyclic hybrids based on quinoline and 1,2,3-triazole. Beilstein J. Org. Chem. 13, 2352–2363 (2017).
Régulier, E., Schneider, B. L., Déglon, N., Beuzard, Y. & Aebischer, P. Continuous delivery of human and mouse erythropoietin in mice by genetically engineered polymer encapsulated myoblasts. Gene Ther. 5, 1014–1022 (1998).
Sommer, B. et al. Long-term doxycycline-regulated secretion of erythropoietin by encapsulated myoblasts. Mol. Ther. 6, 155–161 (2002).
Weber, L. M. & Anseth, K. S. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol. 27, 667–673 (2008).
Suzuki, K. et al. Function and survival of macroencapsulated syngeneic islets transplanted into streptozocin-diabetic mice. Transplantation 66, 21–28 (1998).
Neufeld, T. et al. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS ONE 8, e70150 (2013).
Salehi, S. & Reed, E. F. The divergent roles of macrophages in solid organ transplantation. Curr. Opin. Organ Transpl. 20, 446–453 (2015).
Morris, D. L. Minireview: emerging concepts in islet macrophage biology in type 2 diabetes. Mol. Endocrinol. 29, 946–962 (2015).
Kang, J. W. et al. Combined confocal Raman and quantitative phase microscopy system for biomedical diagnosis. Biomed. Opt. Express 2, 2484–2492 (2011).
Acknowledgements
This work was supported by a JDRF grant (2-SRA-2019-714-S-B) and Leona M. and Harry B. Helmsley Charitable Trust Foundation Grant (2017PG-T1D027) to D.G.A. and R.L. S.B. was supported by the National Institutes of Health and NIBIB (K99EB025254) and a JDRF Postdoctoral Fellowship (PDF-2015-90-A-N). L.R.V. and A.F. were supported by the NSF Graduate Research Fellowship Program. S.J. was supported by the Mazumdar-Shaw oncology fellowship. O.V. was supported by a DOD/CDMRP postdoctoral fellowship (W81XWH-13-1-0215). D.L.G. is supported by the National Institutes of Health (UC4 DK104218). We acknowledge S. K. Aresta-Dasilva, C. Landry and A. Nguyen for assistance with the animal experiments. We also acknowledge S. Hrvatin for discussions. This work was supported in part by the Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute. We thank the Koch Institute Swanson Biotechnology Center for technical support (specifically the Histology, Proteomics and Flow Cytometry core facilities). We also acknowledge the use of resources at the W. M. Keck Biological Imaging Facility and the Genomics core at the Whitehead Institute. This work was performed in part at the Center for Nanoscale Systems, a member of the National Nanotechnology Coordinated Infrastructure network, which is supported by the National Science Foundation under NSF award number 1541959. The Center for Nanoscale Systems is part of Harvard University.
Author information
Authors and Affiliations
Contributions
S.B. and D.G.A. conceived the device, designed the study and wrote the manuscript. S.B., L.R.V., D.T., S.J., C.M., A.W., A.F., Y.T. and C.B. conducted the experiments. V.Y. helped to develop the surface modification method. J.H.-L. and G.C.W. isolated the rat islets and provided technical expertise. O.V., D.L.G. and R.L. provided conceptual advice and technical support. D.G.A. and R.L. supervised the study. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.G.A., R.L. and O.V. are founding scientists of Sigilon Therapeutics—a biotechnology company based in Cambridge, Massachusetts, United States that produces anti-fibrotic materials for cell-based therapies. For a list of entities with which R.L. is involved, compensated or uncompensated, see https://www.dropbox.com/s/yc3xqb5s8s94v7x/Rev%20Langer%20COI.pdf?dl=0.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Table 1 and discussion.
Rights and permissions
About this article
Cite this article
Bose, S., Volpatti, L.R., Thiono, D. et al. A retrievable implant for the long-term encapsulation and survival of therapeutic xenogeneic cells. Nat Biomed Eng 4, 814–826 (2020). https://doi.org/10.1038/s41551-020-0538-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-0538-5
This article is cited by
-
Long-term functional maintenance of primary hepatocytes in vitro using macroporous hydrogels engineered through liquid-liquid phase separation
Nano Research (2024)
-
Electrocatalytic on-site oxygenation for transplanted cell-based-therapies
Nature Communications (2023)
-
Screening hydrogels for antifibrotic properties by implanting cellularly barcoded alginates in mice and a non-human primate
Nature Biomedical Engineering (2023)
-
Controlled swelling of biomaterial devices for improved antifouling polymer coatings
Scientific Reports (2023)
-
Oral formulation of Wnt inhibitor complex reduces inflammation and fibrosis in intraperitoneal implants in vivo
Drug Delivery and Translational Research (2023)