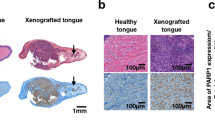
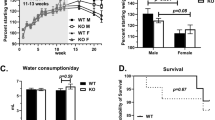
Abstract
For oral, oropharyngeal and oesophageal cancer, the early detection of tumours and of residual tumour after surgery are prognostic factors of recurrence rates and patient survival. Here, we report the validation, in animal models and a human, of the use of a previously described fluorescently labelled small-molecule inhibitor of the DNA repair enzyme poly(ADP–ribose) polymerase 1 (PARP1) for the detection of cancers of the oral cavity, pharynx and oesophagus. We show that the fluorescent contrast agent can be used to quantify the expression levels of PARP1 and to detect oral, oropharyngeal and oesophageal tumours in mice, pigs and fresh human biospecimens when delivered topically or intravenously. The fluorescent PARP1 inhibitor can also detect oral carcinoma in a patient when applied as a mouthwash, and discriminate between fresh biopsied samples of the oral tumour and the surgical resection margin with more than 95% sensitivity and specificity. The PARP1 inhibitor could serve as the basis of a rapid and sensitive assay for the early detection and for the surgical-margin assessment of epithelial cancers of the upper intestinal tract.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the paper and its Supplementary Information files. Associated raw data and step-by-step protocols can be made available from the corresponding author on reasonable request.
Code availability
The ImageJ macro for the automated analysis of PARP1 expression on immunohistochemistry slides is available on reasonable request.
References
Cancer stat facts: oral cavity and pharynx cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program https://seer.cancer.gov/statfacts/html/oralcav.html (2018).
Cancer stat facts: esophageal cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program https://seer.cancer.gov/statfacts/html/esoph.html (2018).
Sutton, D. N., Brown, J. S., Rogers, S. N., Vaughan, E. D. & Woolgar, J. A. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J. Oral Maxillofac. Surg. 32, 30–34 (2003).
Ganly, I., Patel, S. & Shah, J. Early stage squamous cell cancer of the oral tongue—clinicopathologic features affecting outcome. Cancer 118, 101–111 (2012).
Law, S., Arcilla, C., Chu, K. M. & Wong, J. The significance of histologically infiltrated resection margin after esophagectomy for esophageal cancer. Am. J. Surg. 176, 286–290 (1998).
Fedele, S. Diagnostic aids in the screening of oral cancer. Head Neck Oncol. 1, 5 (2009).
Lingen, M. W., Kalmar, J. R., Karrison, T. & Speight, P. M. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 44, 10–22 (2008).
Fuller, C. et al. Adjunctive diagnostic techniques for oral lesions of unknown malignant potential: systematic review with meta-analysis. Head Neck 37, 755–762 (2015).
Olivo, M., Bhuvaneswari, R. & Keogh, I. Advances in bio-optical imaging for the diagnosis of early oral cancer. Pharmaceutics 3, 354–378 (2011).
Strome, A. et al. Current practice and emerging molecular imaging technologies in oral cancer screening. Mol. Imaging 17, 1536012118808644 (2018).
Yang, E. C. et al. Noninvasive diagnostic adjuncts for the evaluation of potentially premalignant oral epithelial lesions: current limitations and future directions. Oral Surg. Oral Med. Oral Pathol. Oral. Radio. 125, 670–681 (2018).
Shin, D., Vigneswaran, N., Gillenwater, A. & Richards-Kortum, R. Advances in fluorescence imaging techniques to detect oral cancer and its precursors. Future Oncol. 6, 1143–1154 (2010).
Patton, L. L., Epstein, J. B. & Kerr, A. R. Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J. Am. Dent. Assoc. 139, 896–905 (2008). quiz 993-894.
Nosho, K. et al. Overexpression of poly(ADP-ribose) polymerase-1 (PARP-1) in the early stage of colorectal carcinogenesis. Eur. J. Cancer 42, 2374–2381 (2006).
Staibano, S. et al. Poly(adenosine diphosphate-ribose) polymerase 1 expression in malignant melanomas from photoexposed areas of the head and neck region. Hum. Pathol. 36, 724–731 (2005).
Chow, J. P. et al. PARP1 is overexpressed in nasopharyngeal carcinoma and its inhibition enhances radiotherapy. Mol. Cancer Therapeutics 12, 2517–2528 (2013).
Salemi, M. et al. Poly (ADP-ribose) polymerase 1 protein expression in normal and neoplastic prostatic tissue. Eur. J. Histochemistry 57, e13 (2013).
Green, A. R. et al. Biological and clinical significance of PARP1 protein expression in breast cancer. Breast Cancer Res. Treat. 149, 353–362 (2015).
Dziaman, T. et al. PARP-1 expression is increased in colon adenoma and carcinoma and correlates with OGG1. PLoS ONE 9, e115558 (2014).
Ossovskaya, V., Koo, I. C., Kaldjian, E. P., Alvares, C. & Sherman, B. M. Upregulation of poly (ADP-Ribose) polymerase-1 (PARP1) in triple-negative breast cancer and other primary human tumour types. Genes Cancer 1, 812–821 (2010).
Michels, J. et al. Negative prognostic value of high levels of intracellular poly(ADP-ribose) in non-small cell lung cancer. Ann. Oncol. 26, 2470–2477 (2015).
Rojo, F. et al. Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann. Oncol. 23, 1156–1164 (2012).
Pashaiefar, H. et al. PARP-1 overexpression as an independent prognostic factor in adult non-M3 acute myeloid leukemia. Genet. Test. Mol. Biomark. 22, 343–349 (2018).
Li, Z. H. et al. PARP1 is a novel independent prognostic factor for the poor prognosis of chordoma. Cancer Biomark. 16, 633–639 (2016).
Irwin, C. P. et al. PARPi-FL—a fluorescent PARP1 inhibitor for glioblastoma imaging. Neoplasia 16, 432–440 (2014).
Kossatz, S. et al. Detection and delineation of oral cancer with a PARP1 targeted optical imaging agent. Sci. Rep. 6, 21371 (2016).
Rosenthal, E. L. et al. Successful translation of fluorescence navigation during oncologic surgery: a consensus report. J. Nucl. Med. 57, 144–150 (2016).
Kariv, R. et al. The Seattle protocol does not more reliably predict the detection of cancer at the time of esophagectomy than a less intensive surveillance protocol. Clin. Gastroenterol. Hepatol. 7, 653–658 (2009).
Canto, M. I. et al. Methylene blue-directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest. Endosc. 51, 560–568 (2000).
Ngamruengphong, S., Sharma, V. K. & Das, A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett’s esophagus: a meta-analysis. Gastrointest. Endosc. 69, 1021–1028 (2009).
Shiozaki, H. et al. Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer 66, 2068–2071 (1990).
Patel, A. A., Strome, M. & Blitzer, A. Directed balloon cytology of the esophagus: a novel device for obtaining circumferential cytologic sampling. Laryngoscope 127, 1032–1035 (2017).
Moinova, H. R. et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci. Transl. Med. 10, eaao5848 (2018).
Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 45, 309–316 (2009).
Johnson, S., Corsten, M. J., McDonald, J. T. & Chun, J. Socio-economic factors and stage at presentation of head and neck cancer patients in Ottawa, Canada: a logistic regression analysis. Oral Oncol. 46, 366–368 (2010).
Conway, D. I. et al. Socioeconomic inequalities and oral cancer risk: a systematic review and meta-analysis of case-control studies. Int. J. Cancer 122, 2811–2819 (2008).
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Mehrotra, R. & Gupta, D. K. Exciting new advances in oral cancer diagnosis: avenues to early detection. Head Neck Oncol. 3, 33 (2011).
Greenberg, M. S. The “brush” controversy. Oral Surg. Oral Med. Oral Pathol. Oral Radio. Endod. 93, 217–218 (2002).
Mehrotra, R. The role of cytology in oral lesions: a review of recent improvements. Diagn. Cytopathol. 40, 73–83 (2012).
Fusaroli, P. et al. Histology vs brush cytology (BC) in the diagnosis and follow up of Barrett’s esophagus (BE). Gastrointest. Endosc. 61, Ab131–Ab131 (2005).
Hardwick, R. H., Morgan, R. J., Warren, B. F., Lott, M. & Alderson, D. Brush cytology in the diagnosis of neoplasia in Barrett’s esophagus. Dis. Esophagus 10, 233–237 (1997).
D’Souza, S. & Addepalli, V. Preventive measures in oral cancer: an overview. Biomed. Pharmacother. 107, 72–80 (2018).
McMahon, J. et al. Influence of condition of surgical margins on local recurrence and disease-specific survival in oral and oropharyngeal cancer. Br. J. Oral Maxillofac. Surg. 41, 224–231 (2003).
Ravasz, L. A., Slootweg, P. J., Hordijk, G. J., Smit, F. & van der Tweel, I. The status of the resection margin as a prognostic factor in the treatment of head and neck carcinoma. J. Craniomaxillofac. Surg. 19, 314–318 (1991).
Alicandri-Ciufelli, M. et al. Surgical margins in head and neck squamous cell carcinoma: what is ‘close’? Eur. Arch. Otorhinolaryngol. 270, 2603–2609 (2013).
Black, C., Marotti, J., Zarovnaya, E. & Paydarfar, J. Critical evaluation of frozen section margins in head and neck cancer resections. Cancer 107, 2792–2800 (2006).
Pathak, K. A. et al. Impact of use of frozen section assessment of operative margins on survival in oral cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radio. Endod. 107, 235–239 (2009).
Kossatz, S., Weber, W. & Reiner, T. Detection and delineation of oral cancer with a PARP1-targeted optical imaging agent. Mol. Imaging 16, 1536012117723786 (2017).
Carney, B., Kossatz, S. & Reiner, T. Molecular Imaging of PARP. J. Nucl. Med. 58, 1025–1030 (2017).
Thurber, G. M., Reiner, T., Yang, K. S., Kohler, R. H. & Weissleder, R. Effect of small-molecule modification on single-cell pharmacokinetics of PARP inhibitors. Mol. Cancer Ther. 13, 986–995 (2014).
van Dam, G. M. et al. Intraoperative tumour-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat. Med. 17, 1315–1319 (2011).
Tummers, W. S. et al. Recommendations for reporting on emerging optical imaging agents to promote clinical approval. Theranostics 8, 5336–5347 (2018).
Carney, B. et al. Target engagement imaging of PARP inhibitors in small-cell lung cancer. Nat. Commun. 9, 176 (2018).
Kossatz, S., Weber, W. A. & Reiner, T. Optical imaging of PARP1 in response to radiation in oral squamous cell carcinoma. PLoS ONE 11, e0147752 (2016).
Kossatz, S. et al. Direct imaging of drug distribution and target engagement of the PARP inhibitor rucaparib. J. Nucl. Med. 59, 1316–1320 (2018).
Acknowledgements
We thank A. Sahu and R. Giese for supporting the work at MSMC; J. Budrewicz for his support of the experiments at CBSet; N. Katabi for providing expertise in histopathology; V. Dokic for assistance during clinical work with PARPi-FL; and G. Scott and L. Bassity for editing the manuscript. We thank the Molecular Cytology Core Facility, Radiochemistry and Molecular Imaging Probes Core Facility, and Flow Cytometry Core Facility at MSKCC. Finally, we thank the participants of the blinded study, including (in alphabetical order): A. Schulman, A. Sahu, A. Bolaender, C. Mason, E. Pratt, C. Andreou, F. Nicolson, J. Berry, J. Goos, J. Gonzales, K. Henry, L. Carter, M. Jain, M. McGill, N. Guru, N. Sobol, P. R. Pereira, R. Mirsafavi, S. Roberts, S. Poty, S. Jannetti, T. Crawford, V. Nagle, X. (L.) Wu and A. Sadique. This work was supported by National Institutes of Health grants R01 CA204441, P30 CA008748, R43 CA228815 and K99 CA218875 (to S.K.). We thank the Tow Foundation and the MSKCC Center for Molecular Imaging and Nanotechnology, Imaging and Radiation Sciences Program, and Molecularly Targeted Intraoperative Imaging Fund.
Author information
Authors and Affiliations
Contributions
S.K., G.P., M.A.K., S.G.P. and T.R. conceived the study and designed the experiments. S.K., G.P., A.L.S., S.P.S., P.D.D.S.F., D.K.Z., C.B., R.A.G., V.S., P.B., N.H., R.D.R., A.S. and T.R. carried out the experiments and collected and analysed the data. S.K., S.P.S., P.D.D.S.F., D.K.Z., A.S., S.G.P. and T.R. wrote Institutional Research Board protocols. A.M., M.G., G.P. and S.K. conducted statistical analysis of the data. M.S. and M.A.K. contributed experimental or analysis tools. S.K. and T.R. wrote the manuscript. All authors carefully reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.K., S.G.P., C.B. and T.R. are shareholders of Summit Biomedical Imaging. S.K., S.G.P. and T.R. are co-inventors on filed US patent (WO2016164771), held by Memorial Sloan Kettering Cancer Centre, which covers methods of use for PARPi-FL. T.R. is a co-inventor on US patent (WO2012074840), held by the General Hospital Corporation, which covers the composition of PARPi-FL. B.C. and T.R. are co-inventors on the filed US patent (WO2016033293), held by Memorial Sloan Kettering Cancer Centre, which covers methods for the synthesis of [18F]PARPi. T.R. is a paid consultant for Theragnostics. M.S. is a co-founder of Aero-Di-Namics.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, tables and references.
Supplementary Dataset 1
Blinded study training dataset.
Supplementary Dataset 2
Blinded study test dataset.
Rights and permissions
About this article
Cite this article
Kossatz, S., Pirovano, G., Demétrio De Souza França, P. et al. Validation of the use of a fluorescent PARP1 inhibitor for the detection of oral, oropharyngeal and oesophageal epithelial cancers. Nat Biomed Eng 4, 272–285 (2020). https://doi.org/10.1038/s41551-020-0526-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-0526-9
This article is cited by
-
PARP1-targeted fluorescence molecular endoscopy as novel tool for early detection of esophageal dysplasia and adenocarcinoma
Journal of Experimental & Clinical Cancer Research (2024)
-
Fluorescence image-guided tumour surgery
Nature Reviews Bioengineering (2023)
-
Polyethylene Glycol 3350 (PEG 3350) as a Practical Vehicle for Rapid Reconstitution of PARPi-FL Formulations for Clinical Use
Molecular Imaging and Biology (2023)
-
New and effective EGFR-targeted fluorescence imaging technology for intraoperative rapid determination of lung cancer in freshly isolated tissue
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Bioorthogonally activatable cyanine dye with torsion-induced disaggregation for in vivo tumor imaging
Nature Communications (2022)