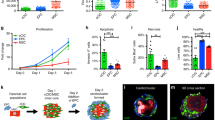
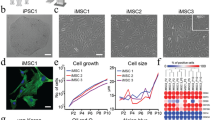
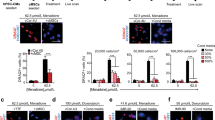
Abstract
Using endogenous mesenchymal stem cells for treating myocardial infarction and other cardiovascular conditions typically results in poor efficacy, in part owing to the heterogeneity of the harvested cells and of the patient responses. Here, by means of high-throughput screening of the combinatorial space of mechanical-strain level and of the presence of particular kinase inhibitors, we show that human mesenchymal stem cells can be mechanically and pharmacologically conditioned to enhance vascular regeneration in vivo. Mesenchymal stem cells conditioned to increase the activation of signalling pathways mediated by Smad2/3 (mothers against decapentaplegic homolog 2/3) and YAP (Yes-associated protein) expressed markers that are associated with pericytes and endothelial cells, displayed increased angiogenic activity in vitro, and enhanced the formation of vasculature in mice after subcutaneous implantation and after implantation in ischaemic hindlimbs. These effects were mediated by the crosstalk of endothelial-growth-factor receptors, transforming-growth-factor-beta receptor type 1 and vascular-endothelial-growth-factor receptor 2. Mechanical and pharmacological conditioning can significantly enhance the regenerative properties of mesenchymal stem cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The source dataset for the RNA-seq analyses can be found in the NIH GEO Database (http://www.ncbi.nlm.nih.gov/bioproject/693356).
References
Sarugaser, R., Hanoun, L., Keating, A., Stanford, W. L. & Davies, J. E. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS ONE 4, e6498 (2009).
Mao, Q. et al. ILK promotes survival and self-renewal of hypoxic MSCs via the activation of lncTCF7-Wnt pathway induced by IL-6/STAT3 signaling. Gene Ther. 26, 165–176 (2019).
Shi, Y. et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 20, 510–518 (2010).
Shake, J. G. et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann. Thorac. Surg. 73, 1919–1925 (2002).
Barbash, I. M. et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108, 863–868 (2003).
Nagaya, N. et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am. J. Physiol. Heart Circ. Physiol. 287, H2670–H2676 (2004).
Muller-Ehmsen, J. et al. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J. Mol. Cell. Cardiol. 41, 876–884 (2006).
Cai, M. et al. PET monitoring angiogenesis of infarcted myocardium after treatment with vascular endothelial growth factor and bone marrow mesenchymal stem cells. Amino Acids 48, 811–820 (2016).
Cai, M. et al. Bone marrow mesenchymal stem cells (BM-MSCs) improve heart function in swine myocardial infarction model through paracrine effects. Sci. Rep. 6, 28250 (2016).
Baraniak, P. R. & McDevitt, T. C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 5, 121–143 (2010).
Cassino, T. R. et al. Mechanical loading of stem cells for improvement of transplantation outcome in a model of acute myocardial infarction: the role of loading history. Tissue Eng. Part A 18, 1101–1108 (2012).
Watt, S. M. et al. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br. Med. Bull. 108, 25–53 (2013).
Yan, J., Tie, G., Xu, T. Y., Cecchini, K. & Messina, L. M. Mesenchymal stem cells as a treatment for peripheral arterial disease: current status and potential impact of type II diabetes on their therapeutic efficacy. Stem Cell Rev. 9, 360–372 (2013).
Wagner, W. et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS ONE 4, e5846 (2009).
Kretlow, J. D. et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 9, 60 (2008).
Phinney, D. G. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J. Cell. Biochem. 113, 2806–2812 (2012).
Du, W. J. et al. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res. Ther. 7, 163 (2016).
Heeschen, C. et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 109, 1615–1622 (2004).
Hill, J. M. et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 348, 593–600 (2003).
Li, T. S. et al. Impaired potency of bone marrow mononuclear cells for inducing therapeutic angiogenesis in obese diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 290, H1362–H1369 (2006).
Roobrouck, V. D., Ulloa-Montoya, F. & Verfaillie, C. M. Self-renewal and differentiation capacity of young and aged stem cells. Exp. Cell Res. 314, 1937–1944 (2008).
Barkholt, L. et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies—bridging scientific observations and regulatory viewpoints. Cytotherapy 15, 753–759 (2013).
Oswald, J. et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22, 377–384 (2004).
Li, Q. et al. VEGF treatment promotes bone marrow-derived CXCR4+ mesenchymal stromal stem cell differentiation into vessel endothelial cells. Exp. Ther. Med. 13, 449–454 (2017).
Janeczek Portalska, K. et al. Endothelial differentiation of mesenchymal stromal cells. PLoS ONE 7, e46842 (2012).
Galas, R. J. Jr. & Liu, J. C. Vascular endothelial growth factor does not accelerate endothelial differentiation of human mesenchymal stem cells. J. Cell. Physiol. 229, 90–96 (2014).
Henderson, K., Sligar, A. D., Le, V., Lee, J. & Baker, A. B. Biomechanical regulation of mesenchymal stem cells for cardiovascular tissue engineering. Adv. Healthc. Mater. 6, 1700556 (2017).
Homayouni Moghadam, F. et al. Treatment with platelet lysate induces endothelial differentation of bone marrow mesenchymal stem cells under fluid shear stress. EXCLI J. 13, 638–649 (2014).
Bai, K., Huang, Y., Jia, X., Fan, Y. & Wang, W. Endothelium oriented differentiation of bone marrow mesenchymal stem cells under chemical and mechanical stimulations. J. Biomech. 43, 1176–1181 (2010).
Dong, J. D. et al. Response of mesenchymal stem cells to shear stress in tissue-engineered vascular grafts. Acta Pharmacol. Sin. 30, 530–536 (2009).
Kim, D. H. et al. Shear stress and circumferential stretch by pulsatile flow direct vascular endothelial lineage commitment of mesenchymal stem cells in engineered blood vessels. J. Mater. Sci. Mater. Med. 27, 60 (2016).
Wang, H. et al. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler. Thromb. Vasc. Biol. 25, 1817–1823 (2005).
Kinnaird, T. et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109, 1543–1549 (2004).
Alaminos, M. et al. Transdifferentiation potentiality of human Wharton’s jelly stem cells towards vascular endothelial cells. J. Cell. Physiol. 223, 640–647 (2010).
Tamama, K., Sen, C. K. & Wells, A. Differentiation of bone marrow mesenchymal stem cells into the smooth muscle lineage by blocking ERK/MAPK signaling pathway. Stem Cells Dev. 17, 897–908 (2008).
Lee, J. et al. A high throughput screening system for studying the effects of applied mechanical forces on reprogramming factor expression. Sci. Rep. 10, 15469 (2020).
Lee, J. & Baker, A. B. Computational analysis of fluid flow within a device for applying biaxial strain to cultured cells. J. Biomech. Eng. 137, 051006 (2015).
Lee, J., Wong, M., Smith, Q. & Baker, A. B. A novel system for studying mechanical strain waveform-dependent responses in vascular smooth muscle cells. Lab Chip 13, 4573–4582 (2013).
Serrano, I., McDonald, P. C., Lock, F., Muller, W. J. & Dedhar, S. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nat. Commun. 4, 2976 (2013).
Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 (2008).
Guimaraes-Camboa, N. et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20, 345–359 (2017).
Xie, L., Zeng, X., Hu, J. & Chen, Q. Characterization of nestin, a selective marker for bone marrow derived mesenchymal stem cells. Stem Cells Int. 2015, 762098 (2015).
Espagnolle, N. et al. CD146 expression on mesenchymal stem cells is associated with their vascular smooth muscle commitment. J. Cell. Mol. Med 18, 104–114 (2014).
Russell, K. C. et al. Cell-surface expression of neuron-glial antigen 2 (NG2) and melanoma cell adhesion molecule (CD146) in heterogeneous cultures of marrow-derived mesenchymal stem cells. Tissue Eng. Part A 19, 2253–2266 (2013).
Wu, C. C., Liu, F. L., Sytwu, H. K., Tsai, C. Y. & Chang, D. M. CD146+ mesenchymal stem cells display greater therapeutic potential than CD146− cells for treating collagen-induced arthritis in mice. Stem Cell Res. Ther. 7, 23 (2016).
Bussolino, F. et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 119, 629–641 (1992).
Kumar, A. et al. Specification and diversification of pericytes and smooth muscle cells from mesenchymoangioblasts. Cell Rep. 19, 1902–1916 (2017).
Bhasin, M. et al. Bioinformatic identification and characterization of human endothelial cell-restricted genes. BMC Genom. 11, 342 (2010).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Sakabe, M. et al. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proc. Natl Acad. Sci. USA 114, 10918–10923 (2017).
Kim, J. et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Invest. 127, 3441–3461 (2017).
Neto, F. et al. YAP and TAZ regulate adherens junction dynamics and endothelial cell distribution during vascular development. eLife 7, 31037 (2018).
Kato, K. et al. Pulmonary pericytes regulate lung morphogenesis. Nat. Commun. 9, 2448 (2018).
Itoh, F. et al. Smad2/Smad3 in endothelium is indispensable for vascular stability via S1PR1 and N-cadherin expressions. Blood 119, 5320–5328 (2012).
Hirschi, K. K., Rohovsky, S. A. & D’Amore, P. A. PDGF, TGF-β, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J. Cell Biol. 141, 805–814 (1998).
Zonneville, J., Safina, A., Truskinovsky, A. M., Arteaga, C. L. & Bakin, A. V. TGF-β signaling promotes tumor vasculature by enhancing the pericyte-endothelium association. BMC Cancer 18, 670 (2018).
Di Bernardini, E. et al. Endothelial lineage differentiation from induced pluripotent stem cells is regulated by microRNA-21 and transforming growth factor β2 (TGF-β2) pathways. J. Biol. Chem. 289, 3383–3393 (2014).
Ai, W. J. et al. R-Smad signaling-mediated VEGF expression coordinately regulates endothelial cell differentiation of rat mesenchymal stem cells. Stem Cells Dev. 24, 1320–1331 (2015).
Choi, H. J. et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat. Commun. 6, 6943 (2015).
Shih, S. C. et al. Transforming growth factor beta1 induction of vascular endothelial growth factor receptor 1: mechanism of pericyte-induced vascular survival in vivo. Proc. Natl Acad. Sci. USA 100, 15859–15864 (2003).
Tsukada, T. et al. TGFβ signaling reinforces pericyte properties of the non-endocrine mouse pituitary cell line TtT/GF. Cell Tissue Res. 371, 339–350 (2018).
Sieczkiewicz, G. J. & Herman, I. M. TGF-β1 signaling controls retinal pericyte contractile protein expression. Microvasc. Res. 66, 190–196 (2003).
Sato, Y. & Rifkin, D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J. Cell Biol. 109, 309–315 (1989).
O’Cearbhaill, E. D. et al. Response of mesenchymal stem cells to the biomechanical environment of the endothelium on a flexible tubular silicone substrate. Biomaterials 29, 1610–1619 (2008).
Jang, J. Y. et al. Combined effects of surface morphology and mechanical straining magnitudes on the differentiation of mesenchymal stem cells without using biochemical reagents. J. Biomed. Biotechnol. 2011, 860652 (2011).
Kim, D. H. et al. Shear stress magnitude is critical in regulating the differentiation of mesenchymal stem cells even with endothelial growth medium. Biotechnol. Lett. 33, 2351–2359 (2011).
Wingate, K., Floren, M., Tan, Y., Tseng, P. O. & Tan, W. Synergism of matrix stiffness and vascular endothelial growth factor on mesenchymal stem cells for vascular endothelial regeneration. Tissue Eng. Part A 20, 2503–2512 (2014).
Dunn, A. K., Bolay, H., Moskowitz, M. A. & Boas, D. A. Dynamic imaging of cerebral blood flow using laser speckle. J. Cereb. Blood Flow Metab. 21, 195–201 (2001).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 4, 1521 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Acknowledgements
We acknowledge funding through the American Heart Association (grant no. 17IRG33410888), the DOD CDMRP (grant nos W81XWH-16-1-0580 and W81XWH-16-1-0582) and the National Institutes of Health (grant nos 1R21EB023551-01, 1R21EB024147-01A1 and 1R01HL141761-01) to A.B.B.
Author information
Authors and Affiliations
Contributions
J.L. and A.B.B. initiated the project and oversaw all aspects of the project. J.L., K.H., M.W.M., M.A.-O., B.G.I., A.V., P.M., E.Y., L.S., M.W. and A.B.B. performed experiments, processed and analysed data. B.-K.L., M.K. and J.K. performed GSEA analyses on the RNA-seq data. A.K.D. provided instrumentation and expertise in laser speckle imaging and data processing. J.L. and A.B.B. wrote and edited the manuscript. All of the authors reviewed and approved the manuscript before publication.
Corresponding author
Ethics declarations
Competing interests
J.L. and A.B.B. have filed a patent (USPTO (US20200268801A1)) on the technology/techniques described in this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Rights and permissions
About this article
Cite this article
Lee, J., Henderson, K., Massidda, M.W. et al. Mechanobiological conditioning of mesenchymal stem cells for enhanced vascular regeneration. Nat Biomed Eng 5, 89–102 (2021). https://doi.org/10.1038/s41551-020-00674-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-00674-w
This article is cited by
-
Exploring the dynamic interplay between exosomes and the immune tumor microenvironment: implications for breast cancer progression and therapeutic strategies
Breast Cancer Research (2024)
-
Therapeutic angiogenesis and tissue revascularization in ischemic vascular disease
Journal of Biological Engineering (2023)
-
CD31 defines a subpopulation of human adipose-derived regenerative cells with potent angiogenic effects
Scientific Reports (2023)
-
Crucial Factors Influencing the Involvement of Odontogenic Exosomes in Dental Pulp Regeneration
Stem Cell Reviews and Reports (2023)
-
High yield engineered nanovesicles from ADSC with enriched miR-21-5p promote angiogenesis in adipose tissue regeneration
Biomaterials Research (2022)