Abstract
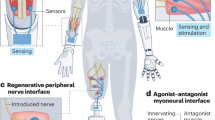
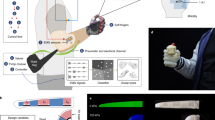
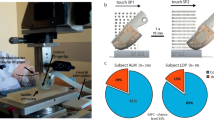
Amputation destroys sensory end organs and does not provide an anatomical interface for cutaneous neuroprosthetic feedback. Here, we report the design and a biomechanical and electrophysiological evaluation of the cutaneous mechanoneural interface consisting of an afferent neural system that comprises a muscle actuator coupled to a natively pedicled skin flap in a cuff-like architecture. Muscle is actuated through electrical stimulation to induce strains or oscillatory vibrations on the skin flap that are proportional to a desired contact duration or contact pressure. In rat hindlimbs, the mechanoneural interface elicited native dermal mechanotransducers to generate at least four levels of graded contact and eight distinct vibratory afferents that were not significantly different from analogous mechanical stimulation of intact skin. The application of different patterns of electrical stimulation independently engaged slowly adapting and rapidly adapting mechanotransducers, and recreated an array of cutaneous sensations. The cutaneous mechanoneural interface can be integrated with current prosthetic technologies for tactile feedback.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the results in this study are available within the paper and its Supplementary Information. The raw data are in a format that is proprietary to the electrophysiology software Synapse, and are available for research purposes from the corresponding authors on reasonable request.
References
Strzalkowski, N. D. J., Peters, R. M., Inglis, J. T. & Bent, L. R. Cutaneous afferent innervation of the human foot sole: what can we learn from single-unit recordings? J. Neurophysiol. 120, 1233–1246 (2018).
Fallon, J. B., Bent, L. R., McNulty, P. A. & Macefield, V. G. Evidence for strong synaptic coupling between single tactile afferents from the sole of the foot and motoneurons supplying leg muscles. J. Neurophysiol. 94, 3795–3804 (2005).
Sainburg, R. L., Ghilardi, M. F., Poizner, H. & Ghez, C. Control of limb dynamics in normal subjects and patients without proprioception. J. Neurophysiol. 73, 820–835 (1995).
Resnik, L. et al. Advanced upper limb prosthetic devices: implications for upper limb prosthetic rehabilitation. Arch. Phys. Med. Rehabil. 93, 710–717 (2012).
Graczyk, E. L., Resnik, L., Schiefer, M. A., Schmitt, M. S. & Tyler, D. J. Home use of a neural-connected sensory prosthesis provides the functional and psychosocial experience of having a hand again. Sci. Rep. 8, 9866 (2018).
Biddiss, E. & Chau, T. Upper-limb prosthetics: critical factors in device abandonment. Am. J. Phys. Med. Rehabil. 86, 977–987 (2007).
Petrini, F. M. et al. Sensory feedback restoration in leg amputees improves walking speed, metabolic cost and phantom pain. Nat. Med. 25, 1356–1363 (2019).
Ortiz-Catalan, M., Håkansson, B. & Brånemark, R. An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs. Sci. Transl. Med. 6, 257re6 (2014).
Page, D. M. et al. Motor control and sensory feedback enhance prosthesis embodiment and reduce phantom pain after long-term hand amputation. Front. Hum. Neurosci. 12, 352 (2018).
Clippinger, F. W., Avery, R. & Titus, B. R. A sensory feedback system for an upper-limb amputation prosthesis. Bull. Prosthet. Res. 247–258 (1974).
Clippinger, F. W., Seaber, A. V., McElhaney, J. H., Harrelson, J. M. & Maxwell, G. M. Afferent sensory feedback for lower extremity prosthesis. Clin. Orthop. Relat. Res. 169, 202–206 (1982).
Markovic, M. et al. The clinical relevance of advanced artificial feedback in the control of a multi-functional myoelectric prosthesis. J. Neuroeng. Rehabil. 15, 28 (2018).
Wendelken, S. et al. Restoration of motor control and proprioceptive and cutaneous sensation in humans with prior upper-limb amputation via multiple Utah slanted electrode arrays (USEAs) implanted in residual peripheral arm nerves. J. Neuroeng. Rehabil. 14, 121 (2017).
Raspopovic, S. et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci. Transl. Med. 6, 222ra19 (2014).
Schiefer, M., Tan, D., Sidek, S. M. & Tyler, D. J. Sensory feedback by peripheral nerve stimulation improves task performance in individuals with upper limb loss using a myoelectric prosthesis. J. Neural Eng. 13, 016001 (2016).
Mastinu, E. et al. Grip control and motor coordination with implanted and surface electrodes while grasping with an osseointegrated prosthetic hand. J. Neuroeng. Rehabil. 16, 49 (2019).
George, J. A. et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot. 4, eaax2352 (2019).
Tan, D. W. et al. A neural interface provides long-term stable natural touch perception. Sci. Transl. Med. 6, 257ra138 (2014).
Ciancio, A. L. et al. Control of prosthetic hands via the peripheral nervous system. Front. Neurosci. 10, 116 (2016).
Dhillon, G. S., Lawrence, S. M., Hutchinson, D. T. & Horch, K. W. Residual function in peripheral nerve stumps of amputees: implications for neural control of artificial limbs. J. Hand Surg. 29, 605–615 (2004).
Alahakone, A. U. & Senanayake, S. M. N. A. Vibrotactile feedback systems: current trends in rehabilitation, sports and information display. In Proc. 2009 IEEE/ASME International Conference on Advanced Intelligent Mechatronics 1148–1153 https://doi.org/10.1109/AIM.2009.5229741 (IEEE, 2009).
Hebert, J. S. et al. Novel targeted sensory reinnervation technique to restore functional hand sensation after transhumeral amputation. IEEE Trans. Neural Syst. Rehabil. Eng. 22, 765–773 (2014).
Marasco, P. D., Schultz, A. E. & Kuiken, T. A. Sensory capacity of reinnervated skin after redirection of amputated upper limb nerves to the chest. Brain 132, 1441–1448 (2009).
Hebert, J. S., Elzinga, K., Chan, K. M., Olson, J. & Morhart, M. Updates in targeted sensory reinnervation for upper limb amputation. Curr. Surg. Rep. 2, 45 (2014).
Clemente, F., D’Alonzo, M., Controzzi, M., Edin, B. B. & Cipriani, C. Non-invasive, temporally discrete feedback of object contact and release improves grasp control of closed-loop myoelectric transradial prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 24, 1314–1322 (2016).
Ruhe, A., Fejer, R. & Walker, B. Center of pressure excursion as a measure of balance performance in patients with non-specific low back pain compared to healthy controls: a systematic review of the literature. Eur. Spine J. 20, 358–368 (2011).
Lugade, V. & Kaufman, K. Center of pressure trajectory during gait: a comparison of four foot positions. Gait Posture 40, 252–254 (2014).
Sardain, P. & Bessonnet, G. Forces acting on a biped robot. Center of pressure-zero moment point. IEEE Trans. Syst. Man Cybern. A Syst. Hum. 34, 630–637 (2004).
Herr, H. M., Riso, R. R., Song, K. W., Casler Jr, R. J. & Carty, M. J. Peripheral neural interface via nerve regeneration to distal tissues. US patent US9474634B2 (2016).
Ziegler-Graham, K., MacKenzie, E. J., Ephraim, P. L., Travison, T. G. & Brookmeyer, R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 89, 422–429 (2008).
Pasquina, P. F. et al. Special considerations for multiple limb amputation. Curr. Phys. Med. Rehabil. Rep. 2, 273–289 (2014).
Herr, H. M. et al. Reinventing extremity amputation in the era of functional limb restoration. Ann. Surg. https://doi.org/10.1097/SLA.0000000000003895 (2020).
Geiss, L. S. et al. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care 42, 50–54 (2019).
Zimmerman, A., Bai, L. & Ginty, D. D. The gentle touch receptors of mammalian skin. Science 346, 950–954 (2014).
Walcher, J. et al. Specialized mechanoreceptor systems in rodent glabrous skin: glabrous skin mechanoreceptors. J. Physiol. 596, 4995–5016 (2018).
Jenkins, B. A. & Lumpkin, E. A. Developing a sense of touch. Development 144, 4078–4090 (2017).
Hahn, J. M. et al. Identification of Merkel cells associated with neurons in engineered skin substitutes after grafting to full thickness wounds. PLoS ONE 14, e0213325 (2019).
Fleming, M. S. & Luo, W. The anatomy, function, and development of mammalian Aβ low-threshold mechanoreceptors. Front. Biol. 8, 4 (2013).
Coleman, G. T., Bahramali, H., Zhang, H. Q. & Rowe, M. J. Characterization of tactile afferent fibers in the hand of the marmoset monkey. J. Neurophysiol. 85, 1793–1804 (2001).
Fitzgerald, M. Cutaneous primary afferent properties in the hind limb of the neonatal rat. J. Physiol. 383, 79–92 (1987).
Yu, X. et al. Skin-integrated wireless haptic interfaces for virtual and augmented reality. Nature 575, 473–479 (2019).
Casal, D. et al. A model of free tissue transfer: the rat epigastric free flap. J. Vis. Exp. https://doi.org/10.3791/55281 (2017).
Nissen, T. D. et al. Translational aspects of rectal evoked potentials: a comparative study in rats and humans. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G119–G128 (2013).
Srinivasan, S. S. et al. On prosthetic control: a regenerative agonist-antagonist myoneural interface. Sci. Robot. 2, eaan2971 (2017).
Srinivasan, S. S., Diaz, M., Carty, M. & Herr, H. M. Towards functional restoration for persons with limb amputation: a dual-stage implementation of regenerative agonist-antagonist myoneural interfaces. Sci. Rep. 9, 1981 (2019).
Kung, T. A. et al. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast. Reconstr. Surg. 133, 1380–1394 (2014).
Kubiak, C. A., Kemp, S. W. P., Cederna, P. S. & Kung, T. A. Prophylactic regenerative peripheral nerve interfaces to prevent postamputation pain. Plast. Reconstr. Surg. 144, 421e–430e (2019).
Armiger, R. S. et al. Enabling closed-loop control of the modular prosthetic limb through haptic feedback. Johns. Hopkins APL Tech. Dig. 31, 345–353 (2013).
Haslinger, G. The grip-stabilising-sensor ‘an example for integrating miniaturized sensorics into a myo-electric hand’. In MEC 97 Proc. 1997 MyoElectric Controls/Powered Prosthetics Symposium (1997).
Osborn, L. E. et al. Prosthesis with neuromorphic multilayered e-dermis perceives touch and pain. Sci. Robot. 3, eaat3818 (2018).
Rossini, P. M. et al. Double nerve intraneural interface implant on a human amputee for robotic hand control. Clin. Neurophysiol. 121, 777–783 (2010).
Arakawa, T. et al. Electrical stimulation prevents apoptosis in denervated skeletal muscle. NeuroRehabilitation 27, 147–154 (2010).
Eberstein, A. & Eberstein, S. Electrical stimulation of denervated muscle: is it worthwhile? Med. Sci. Sports Exerc. 28, 1463–1469 (1996).
La, G. et al. Proteomics and transcriptomics analysis reveals clues into the mechanism of the beneficial effect of electrical stimulation on rat denervated gastrocnemius muscle. Cell. Physiol. Biochem. 52, 769–786 (2019).
Dow, D. E., Dennis, R. G. & Faulkner, J. A. Electrical stimulation attenuates denervation and age-related atrophy in extensor digitorum longus muscles of old rats. J. Gerontol. A 60, 416–424 (2005).
Nghiem, B. T., Sando, I. C., Hu, Y., Urbanchek, M. G. & Cederna, P. S. Sensory protection to enhance functional recovery following proximal nerve injuries: current trends. Plast. Aesthet. Res. https://doi.org/10.4103/2347-9264.156982 (2015).
Irwin, Z. T. et al. Chronic recording of hand prosthesis control signals via a regenerative peripheral nerve interface in a rhesus macaque. J. Neural Eng. 13, 046007 (2016).
Kolasinski, J. et al. Perceptually relevant remapping of human somatotopy in 24 hours. eLife 5, e17280 (2016).
Dempsey-Jones, H. et al. Transfer of tactile perceptual learning to untrained neighboring fingers reflects natural use relationships. J. Neurophysiol. 115, 1088–1097 (2015).
Granata, G. et al. ID 287—sensory feedback generated by intraneural electrical stimulation of peripheral nerves drives cortical reorganization and relieves phantom limb pain: a case study. Clin. Neurophysiol. 127, e63 (2016).
Di Pino, G., Guglielmelli, E. & Rossini, P. M. Neuroplasticity in amputees: main implications on bidirectional interfacing of cybernetic hand prostheses. Prog. Neurobiol. 88, 114–126 (2009).
Serino, A. et al. Upper limb cortical maps in amputees with targeted muscle and sensory reinnervation. Brain 140, 2993–3011 (2017).
Luis-Delgado, O. E. et al. Calibrated forceps: a sensitive and reliable tool for pain and analgesia studies. J. Pain 7, 32–39 (2006).
Acknowledgements
We thank V. Shah, R. Rodarte and H. G. Song for their assistance in animal surgeries. This work was funded by the MIT Media Lab Consortium.
Author information
Authors and Affiliations
Contributions
S.S.S. conceptualized the CMI, performed the surgeries, data collection, analysis and writing of the manuscript. H.M.H. conceptualized the CMI, provided project management, contributed to the experimental design and manuscript preparations.
Corresponding authors
Ethics declarations
Competing interests
The authors are inventors on patents (United States application no. 63/029,137) that describe the CMI.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Discussion and Figs. 1–10.
Supplementary Video 1
Stimulation at 12 mA producing maximal contractions of the skin flap.
Rights and permissions
About this article
Cite this article
S. Srinivasan, S., M. Herr, H. A cutaneous mechanoneural interface for neuroprosthetic feedback. Nat. Biomed. Eng 6, 731–740 (2022). https://doi.org/10.1038/s41551-020-00669-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-00669-7
This article is cited by
-
Mechanoneural interfaces for bionic integration
Nature Reviews Bioengineering (2024)
-
Stretchable surface electromyography electrode array patch for tendon location and muscle injury prevention
Nature Communications (2023)
-
Evoking natural thermal perceptions using a thin-film thermoelectric device with high cooling power density and speed
Nature Biomedical Engineering (2023)
-
Multichannel haptic feedback unlocks prosthetic hand dexterity
Scientific Reports (2022)
-
Recent trends in bioartificial muscle engineering and their applications in cultured meat, biorobotic systems and biohybrid implants
Communications Biology (2022)