Abstract
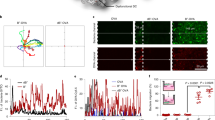
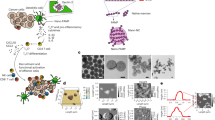
Eliciting immune responses against primary tumours is hampered by their immunosuppressive microenvironment and by the greater inaccessibility of deeper intratumoural cells. However, metastatic tumour cells are exposed to highly perfused and immunoactive organs, such as the lungs. Here, by taking advantage of the preferential colocalization of intravenously administered erythrocytes with metastases in the lungs, we show that treatment with chemokine-encapsulating nanoparticles that are non-covalently anchored onto the surface of injected erythrocytes results in local and systemic tumour suppression in mouse models of lung metastasis. Such erythrocyte-anchored systemic immunotherapy led to the infiltration of effector immune cells into the lungs, in situ immunization without the need for exogenous antigens, inhibition of the progression of lung metastasis, and significantly extended animal survival and systemic immunity that suppressed the growth of distant tumours after rechallenge. Erythrocyte-mediated systemic immunotherapy may represent a general and potent strategy for cancer vaccination.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding author on reasonable request.
References
Restifo, N. P., Dudley, M. E. & Rosenberg, S. A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281 (2012).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Rosenberg, S. A. Decade in review-cancer immunotherapy: entering the mainstream of cancer treatment. Nat. Rev. Clin. Oncol. 11, 630–632 (2014).
Qian, Y. et al. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumour-associated macrophages. ACS Nano 11, 9536–9549 (2017).
Riley, R. S., June, C. H., Langer, R. & Mitchell, M. J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 18, 175–196 (2019).
Shah, N. J. et al. A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. Nat. Biomed. Eng. 4, 40–51 (2020).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Fesnak, A. D., June, C. H. & Levine, B. L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 16, 566–581 (2016).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Mellman, I., Coukos, G. & Dranoff, G. Cancer immunotherapy comes of age. Nature 480, 480–489 (2011).
Nagarsheth, N., Wicha, M. S. & Zou, W. P. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 17, 559–572 (2017).
Vijayan, D., Young, A., Teng, M. W. L. & Smyth, M. J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 17, 709–724 (2017).
Chauhan, V. P. et al. Reprogramming the microenvironment with tumour-selective angiotensin blockers enhances cancer immunotherapy. Proc. Natl Acad. Sci. USA 116, 10674–10680 (2019).
Gupta, G. P. & Massague, J. Cancer metastasis: building a framework. Cell 127, 679–695 (2006).
Schroeder, A. et al. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer 12, 39–50 (2011).
Metastatic cancer. National Cancer Institute https://www.cancer.gov/types/metastatic-cancer#where-cancer-spreads (2019).
Cardoso, F. et al. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 21, VII11–VII19 (2010).
Eckhardt, B. L., Francis, P. A., Parker, B. S. & Anderson, R. L. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat. Rev. Drug Discov. 11, 479–497 (2012).
Homey, B., Muller, A. & Zlotnik, A. Chemokines: agents for the immunotherapy of cancer? Nat. Rev. Immunol. 2, 175–184 (2002).
Clancy-Thompson, E. et al. Melanoma induces, and adenosine suppresses, CXCR3-cognate chemokine production and T-cell infiltration of lungs bearing metastatic-like disease. Cancer Immunol. Res. 3, 956–967 (2015).
Altorki, N. K. et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat. Rev. Cancer 19, 9–31 (2019).
Zhao, Z., Ukidve, A., Gao, Y., Kim, J. & Mitragotri, S. Erythrocyte leveraged chemotherapy (ELeCt): nanoparticle assembly on erythrocyte surface to combat lung metastasis. Sci. Adv. 5, eaax9250 (2019).
Anselmo, A. C. et al. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano 7, 11129–11137 (2013).
Makadia, H. K. & Siegel, S. J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3, 1377–1397 (2011).
Paszkowiak, J. J. & Dardik, A. Arterial wall shear stress: observations from the bench to the bedside. Vasc. Endovasc. Surg. 37, 47–57 (2003).
Chatterjee, S. Endothelial mechanotransduction, redox signaling and the regulation of vascular inflammatory pathways. Front. Physiol. 9, 524 (2018).
Malek, A. M., Alper, S. L. & Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282, 2035–2042 (1999).
Brenner, J. S. et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 9, 2684 (2018).
Muro, S. et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther. 16, 1450–1458 (2008).
Scherpereel, A. et al. Cell-selective intracellular delivery of a foreign enzyme to endothelium in vivo using vascular immunotargeting. FASEB J. 15, 416–426 (2001).
Guo, P. et al. ICAM-1 as a molecular target for triple negative breast cancer. Proc. Natl Acad. Sci. USA 111, 14710–14715 (2014).
Guo, P. et al. Dual complementary liposomes inhibit triple-negative breast tumour progression and metastasis. Sci. Adv. 5, eaav5010 (2019).
Lee, S. J., Park, S. Y., Jung, M. Y., Bae, S. M. & Kim, I. S. Mechanism for phosphatidylserine-dependent erythrophagocytosis in mouse liver. Blood 117, 5215–5223 (2011).
Oldenborg, P. A. et al. Role of CD47 as a marker of self on red blood cells. Science 288, 2051–2054 (2000).
Pan, D. C. et al. Nanoparticle properties modulate their attachment and effect on carrier red blood cells. Sci. Rep. 8, 1615 (2018).
Ukidve, A. et al. Erythrocyte-driven immunization via biomimicry of their natural antigen-presenting function. Proc. Natl Acad. Sci. USA 117, 17727–17736 (2020).
Tokunaga, R. et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—a target for novel cancer therapy. Cancer Treat. Rev. 63, 40–47 (2018).
Hu, J. M. et al. CXCL9, CXCL10 and IFNγ favor the accumulation of infused T cells in tumours following IL-12 plus doxorubicin treatment. J. Immunol. 196, 212.1 (2016).
Pfirschke, C., Siwicki, M., Liao, H. W. & Pittet, M. J. Tumour microenvironment: no effector T cells without dendritic cells. Cancer Cell 31, 614–615 (2017).
Zhu, J. F. & Paul, W. E. CD4 T cells: fates, functions, and faults. Blood 112, 1557–1569 (2008).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Hsu, J. et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Invest. 128, 4654–4668 (2018).
Vivier, E., Ugolini, S., Blaise, D., Chabannon, C. & Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 12, 239–252 (2012).
Binnewies, M. et al. Understanding the tumour immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Wang, H. & Mooney, D. J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 17, 761–772 (2018).
Bottcher, J. P. et al. NK cells stimulate recruitment of cDC1 into the tumour microenvironment promoting cancer immune control. Cell 172, 1022–1037 (2018).
Barry, K. C. et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumour microenvironments. Nat. Med. 24, 1178–1191 (2018).
Harizi, H. Reciprocal crosstalk between dendritic cells and natural killer cells under the effects of PGE2 in immunity and immunopathology. Cell. Mol. Immunol. 10, 213–221 (2013).
Nguyen, K. B. & Spranger, S. Modulation of the immune microenvironment by tumour-intrinsic oncogenic signaling. J. Cell Biol. 219, e201908224 (2020).
Schmid, D. et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumour immunity. Nat. Commun. 8, 1747 (2017).
Tang, L. et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 36, 707–716 (2018).
Stephan, M. T., Moon, J. J., Um, S. H., Bershteyn, A. & Irvine, D. J. Therapeutic cell engineering using surface-conjugated synthetic nanoparticles. J. Immunother. 33, 866–866 (2010).
Kontos, S., Kourtis, I. C., Dane, K. Y. & Hubbell, J. A. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc. Natl Acad. Sci. USA 110, E60–E68 (2013).
Lorentz, K. M., Kontos, S., Diaceri, G., Henry, H. & Hubbell, J. A. Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase. Sci. Adv. 1, e1500112 (2015).
Masuda, Y., Murata, Y., Hayashi, M. & Nanba, H. Inhibitory effect of MD-fraction on tumour metastasis: involvement of NK cell activation and suppression of intercellular adhesion molecule (ICAM)-1 expression in lung vascular endothelial cells. Biol. Pharm. Bull. 31, 1104–1108 (2008).
Cambien, B. et al. Organ-specific inhibition of metastatic colon carcinoma by CXCR3 antagonism. Br. J. Cancer 100, 1755–1764 (2009).
Zhu, G. Q. et al. CXCR3 as a molecular target in breast cancer metastasis: inhibition of tumour cell migration and promotion of host anti-tumour immunity. Oncotarget 6, 43408–43419 (2015).
Ma, X. R. et al. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol. Cancer Ther. 8, 490–498 (2009).
Acknowledgements
We thank D. Mooney for guidance. This work was financially supported by the Wyss Institute at Harvard University. The authors acknowledge funding from the National Institutes of Health (grant no. 1R01HL143806-01).
Author information
Authors and Affiliations
Contributions
Z.Z., A.U. and S.M. conceived and designed the experiments. Z.Z., A.U., V.K., A.F., D.C.P., Y.G., J.K., A.M., J.G. and M.A.E. performed the experiments. Z.Z., A.U., V.K., A.F., D.C.P., Y.G., J.K., A.M., J.G., M.A.E., V.M. and S.M. analysed and discussed results. Z.Z., A.U. and S.M. wrote the manuscript. All of the authors read, revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.M., A.U. and Z.Z. are inventors on a patent application with aspects related to this work filed by Harvard University (no. 62/858,478).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–39, Table 1, methods, discussion and references.
Rights and permissions
About this article
Cite this article
Zhao, Z., Ukidve, A., Krishnan, V. et al. Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases. Nat Biomed Eng 5, 441–454 (2021). https://doi.org/10.1038/s41551-020-00644-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-00644-2
This article is cited by
-
Neutrophils bearing adhesive polymer micropatches as a drug-free cancer immunotherapy
Nature Biomedical Engineering (2024)
-
Cell-mediated nanoparticle delivery systems: towards precision nanomedicine
Drug Delivery and Translational Research (2024)
-
Modularity of RBC hitchhiking with polymeric nanoparticles: testing the limits of non-covalent adsorption
Journal of Nanobiotechnology (2022)
-
Red Blood Cell Inspired Strategies for Drug Delivery: Emerging Concepts and New Advances
Pharmaceutical Research (2022)
-
Biomedical polymers: synthesis, properties, and applications
Science China Chemistry (2022)