Abstract
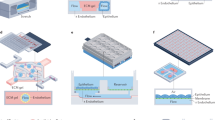
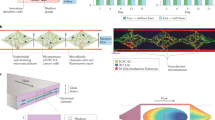
Analyses of drug pharmacokinetics (PKs) and pharmacodynamics (PDs) performed in animals are often not predictive of drug PKs and PDs in humans, and in vitro PK and PD modelling does not provide quantitative PK parameters. Here, we show that physiological PK modelling of first-pass drug absorption, metabolism and excretion in humans—using computationally scaled data from multiple fluidically linked two-channel organ chips—predicts PK parameters for orally administered nicotine (using gut, liver and kidney chips) and for intravenously injected cisplatin (using coupled bone marrow, liver and kidney chips). The chips are linked through sequential robotic liquid transfers of a common blood substitute by their endothelium-lined channels (as reported by Novak et al. in an associated Article) and share an arteriovenous fluid-mixing reservoir. We also show that predictions of cisplatin PDs match previously reported patient data. The quantitative in-vitro-to-in-vivo translation of PK and PD parameters and the prediction of drug absorption, distribution, metabolism, excretion and toxicity through fluidically coupled organ chips may improve the design of drug-administration regimens for phase-I clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the results in this study are available within the Article and its Supplementary Information. The broad range of raw datasets acquired and analysed (or any subsets of it), which for reuse would require contextual metadata, are available from the corresponding author on reasonable request.
Code availability
The CoBi code used to simulate individual organs and their network, as well as individual organ models, is freely available at http://medicalavatars.cfdrc.com/index.php/cobi-tools, under the folder ‘Microphysiological Organs and Systems Models’.
References
Shanks, N., Greek, R. & Greek, J. Are animal models predictive for humans? Philos. Ethics Humanit. Med. 4, 2 (2009).
Malinowski, H. et al. Draft guidance for industry extended-release solid oral dosage forms. Development, evaluation and application of in vitro-in vivo correlations. Adv. Exp. Med. Biol. 423, 269–288 (1997).
Danhof, M., de Lange, E. C. M., Della Pasqua, O. E., Ploeger, B. A. & Voskuyl, R. A. Mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modeling in translational drug research. Trends Pharmacol. Sci. 29, 186–191 (2008).
Abaci, H. E. & Shuler, M. L. Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr. Biol. 7, 383–391 (2015).
Esch, M. B., Ueno, H., Applegate, D. R. & Shuler, M. L. Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue. Lab Chip 16, 2719–2729 (2016).
Coppeta, J. R. et al. A portable and reconfigurable multi-organ platform for drug development with onboard microfluidic flow control. Lab Chip 17, 134–144 (2016).
Xiao, S. et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 8, 14584 (2017).
Wagner, I. et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 13, 3538–3547 (2013).
Stokes, C. L., Cirit, M. & Lauffenburger, D. A. Physiome-on-a-Chip: the challenge of “scaling” in design, operation, and translation of microphysiological systems. CPT Pharmacomet. Pharmacol. 4, 559–562 (2015).
Bovard, D. et al. A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab Chip 18, 3814–3829 (2018).
Oleaga, C. et al. Multi-organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci. Rep. 6, 20030 (2016).
Maschmeyer, I. et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 15, 2688–2699 (2015).
Edington, C. D. et al. Interconnected microphysiological systems for quantitative biology and pharmacology Studies. Sci. Rep. 8, 4530 (2018).
Vernetti, L. et al. Functional coupling of human microphysiology systems: intestine, liver, kidney proximal tubule, blood-brain barrier and skeletal muscle. Sci. Rep. 7, 42296 (2017).
Kim, H. J., Li, H., Collins, J. J. & Ingber, D. E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl Acad. Sci. USA 113, E7–E15 (2016).
Novak, R. et al. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-019-0497-x (2020).
Jang, K.-J. et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 5, 1119–1129 (2013).
Prantil-Baun, R. et al. Physiologically based pharmacokinetic and pharmacodynamic analysis enabled by microfluidically linked organs-on-chips. Annu. Rev. Pharmacol. Toxicol. 58, 37–64 (2018).
Auner, A. W., Tasneem, K. M., Markov, D. A., McCawley, L. J. & Hutson, M. S. Chemical-PDMS binding kinetics and implications for bioavailability in microfluidic devices. Lab Chip 19, 864–874 (2019).
Jalili-Firoozinezhad, S. et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 3, 520–531 (2019).
Kasendra, M. et al. Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci. Rep. 8, 2871 (2018).
Kim, H. J., Huh, D., Hamilton, G. & Ingber, D. E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12, 2165–2174 (2012).
Jang, K.-J. et al. Reproducing human and cross-species toxicities using a Liver-Chip. Science Transl. Med. 11, eaax5516 (2019).
Pullan, R. D. et al. Transdermal nicotine for active ulcerative colitis. N. Engl. J. Med. 330, 811–815 (1994).
Benowitz, N. L., Hukkanen, J. & Jacob, P. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 192, 29–60 (2009).
Dancik, Y., Anissimov, Y. G., Jepps, O. G. & Roberts, M. S. Convective transport of highly plasma protein bound drugs facilitates direct penetration into deep tissues after topical application. Br. J. Clin. Pharm. 73, 564–578 (2012).
Varma, M. V. S. et al. Physicochemical determinants of human renal clearance. J. Med. Chem. 52, 4844–4852 (2009).
Digard, H., Proctor, C., Kulasekaran, A., Malmqvist, U. & Richter, A. Determination of nicotine absorption from multiple tobacco products and nicotine gum. Nicotine Tob. Res. 15, 255–261 (2013).
Prytz, H., Benoni, C. & Tagesson, C. Does smoking tighten the gut? Scand. J. Gastroenterol. 24, 1084–1088 (1989).
Suenaert, P. et al. In vivo influence of nicotine on human basal and NSAID-induced gut barrier function. Scand. J. Gastroenterol. 38, 399–408 (2003).
McGilligan, V. E., Wallace, J. M. W., Heavey, P. M., Ridley, D. L. & Rowland, I. R. The effect of nicotine in vitro on the integrity of tight junctions in Caco-2 cell monolayers. Food Chem. Toxicol. 45, 1593–1598 (2007).
Rodriguez-Gaztelumendi, A., Alvehus, M., Andersson, T. & Jacobsson, S. O. P. Comparison of the effects of nicotine upon the transcellular electrical resistance and sucrose permeability of human ECV304/rat C6 co-cultures and human CaCo2 cells. Toxicol. Lett. 207, 1–6 (2011).
Jones, H. M. & Rowland-Yeo, K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacomet. Pharmacol. 2, 1–12 (2013).
Yamazaki, H. et al. Human blood concentrations of cotinine, a biomonitoring marker for tobacco smoke, extrapolated from nicotine metabolism in rats and humans and physiologically based pharmacokinetic modeling. Int. J. Environ. Res. Publ. Health 7, 3406–3421 (2010).
Hartmann, J. T. & Lipp, H.-P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 4, 889–901 (2003).
Chou, D. B. et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-019-0495-z (2020).
Sparreboom, A., Nooter, K., Loos, W. J. & Verweij, J. The (ir)relevance of plasma protein binding of anticancer drugs. Neth. J. Med. 59, 196–207 (2001).
Rajkumar, P. et al. Cisplatin concentrations in long and short duration infusion: implications for the optimal time of radiation delivery. J. Clin. Diagn. Res. 10, XC01–XC04 (2016).
Wikswo, J. P. et al. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab Chip 13, 3496–3511 (2013).
Maass, C., Stokes, C. L., Griffith, L. G. & Cirit, M. Multi-functional scaling methodology for translational pharmacokinetic and pharmacodynamic applications using integrated microphysiological systems (MPS). Integr. Biol. 9, 290–302 (2017).
Neault, J. F. & Tajmir-Riahi, H. A. Interaction of cisplatin with human serum albumin. Drug binding mode and protein secondary structure. Biochim. Biophys. Acta 1384, 153–159 (1998).
Vickers, A. E. M. et al. Kidney slices of human and rat to characterize cisplatin-induced injury on cellular pathways and morphology. Toxicol. Pathol. 32, 577–590 (2004).
Huang, Q. et al. Assessment of cisplatin-induced nephrotoxicity by microarray technology. Toxicol. Sci. 63, 196–207 (2001).
Maass, C. et al. Establishing quasi-steady state operations of microphysiological systems (MPS) using tissue-specific metabolic dependencies. Sci. Rep. 8, 8015 (2018).
Huh, D. et al. Microfabrication of human organs-on-chips. Nat. Protoc. 8, 2135–2157 (2013).
Park, T. -E. et al. Hypoxia-enhanced blood-brain barrier chip recapitulates human barrier function, drug penetration, and antibody shuttling properties. Nat. Commun. 10, 2621 (2019).
Elamin, E. E. et. al. in Molecular Aspects of Alcohol and Nutrition: A Volume in the Molecular Nutrition Series (ed. Patel, V. B.) Ch. 14 (Elsevier, 2016).
Henry, O. Y. F. et al. Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab Chip 17, 2264–2271 (2017).
Maoz, B. M. et al. Organs-on-Chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip 17, 2294–2302 (2017).
Przekwas, A., Friend, T., Teixeira, R., Chen, Z. & Wilkerson, P. Spatial Modeling Tools for Cell Biology (Air Force Research Laboratory Information Directorate Rome Research Site, 2006).
Adams, B. M. et al. Dakota, a Multilevel Parallel Object-Oriented Framework for Design Optimization, Parameter Estimation, Uncertainty Quantification, and Sensitivity Analysis (OSTI, 2014).
Amidon, G. L., Lennernäs, H., Shah, V. P. & Crison, J. R. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12, 413–420 (1995).
O’Hara, T. et al. In vivo-in vitro correlation (IVIVC) modeling incorporating a convolution step. J. Pharmacokinet. Pharmacodyn. 28, 277–298 (2001).
Howell, B. A. et al. In vitro to in vivo extrapolation and species response comparisons for drug-induced liver injury (DILI) using DILIsymTM: a mechanistic, mathematical model of DILI. J. Pharmacokinet. Pharmacodyn. 39, 527–541 (2012).
Poulin, P. & Haddad, S. Toward a new paradigm for the efficient in vitro-in vivo extrapolation of metabolic clearance in humans from hepatocyte data. J. Pharm. Sci. 102, 3239–3251 (2013).
Chen, Y., Jin, J. Y., Mukadam, S., Malhi, V. & Kenny, J. R. Application of IVIVE and PBPK modeling in prospective prediction of clinical pharmacokinetics: strategy and approach during the drug discovery phase with four case studies. Biopharm. Drug Dispos. 33, 85–98 (2012).
Rostami-Hodjegan, A. Physiologically based pharmacokinetics joined with in vitro-in vivo extrapolation of ADME: a marriage under the arch of systems pharmacology. Clin. Pharmacol. Ther. 92, 50–61 (2012).
Cirit, M. & Stokes, C. L. Maximizing the impact of microphysiological systems with in vitro-in vivo translation. Lab Chip 18, 1831–1837 (2018).
Zhu, C., Jiang, L., Chen, T.-M. & Hwang, K.-K. A comparative study of artificial membrane permeability assay for high throughput profiling of drug absorption potential. Eur. J. Med. Chem. 37, 399–407 (2002).
Hukkanen, J., Jacob, P. & Benowitz, N. L. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 57, 79–115 (2005).
Riley, R. J., McGinnity, D. F. & Austin, R. P. A unified model for predicting human hepatic, metabolic clearance from in vitro intrinsic clearance data in hepatocytes and microsomes. Drug Metab. Dispos. 33, 1304–1311 (2005).
Chiba, M., Ishii, Y. & Sugiyama, Y. Prediction of hepatic clearance in human from in vitro data for successful drug development. AAPS J. 11, 262–276 (2009).
Jamei, M. et al. A mechanistic framework for in vitro-in vivo extrapolation of liver membrane transporters: prediction of drug-drug interaction between rosuvastatin and cyclosporine. Clin. Pharmacokinet. 53, 73–87 (2014).
Sluka, J. P. et al. A liver-centric multiscale modeling framework for xenobiotics. PLoS ONE 11, e0162428 (2016).
Clancy, C. E. et al. Multiscale modeling in the clinic: drug design and development. Ann. Biomed. Eng. 44, 2591–2610 (2016).
Kannan, R. R., Singh, N. & Przekwas, A. A compartment-quasi-3D multiscale approach for drug absorption, transport, and retention in the human lungs. Int. J. Numer. Method Biomed. Eng. 34, e2955 (2018).
Tsamandouras, N. et al. Integrated gut and liver microphysiological systems for quantitative in vitro pharmacokinetic studies. AAPS J. 19, 1499–1512 (2017).
Gong, C. et al. Hepatotoxicity and pharmacokinetics of cisplatin in combination therapy with a traditional Chinese medicine compound of Zengmian Yiliu granules in ICR mice and SKOV-3-bearing nude mice. BMC Complement. Altern. Med. 15, 283 (2015).
Acknowledgements
We thank M. Rosnach for his artwork, B. Fountaine and S. Kroll for their help with photography, N. Dimitrakakis for statistical analysis and C. Vidoudez for MS analysis. This research was sponsored by the Wyss Institute for Biologically Inspired Engineering at Harvard University, the Defense Advanced Research Projects Agency under cooperative agreement no. W911NF-12-2-0036 and US Food and Drug Administration grant HHSF223201310079C. Funding from Knut och Alice Wallenberg’s stiftelse (grant no. 2015-0178; to A.H.) is acknowledged. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Defense Advanced Research Projects Agency, or the US Government. This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Coordinated Infrastructure Network, which is supported by the National Science Foundation under award no. 1541959. The CNS is part of Harvard University, the Harvard Materials Research Science and Engineering Center (DMR-1420570).
Author information
Authors and Affiliations
Contributions
A.H., B.M.M., R.N. and R.P.-B. helped to design and manage the multi-organ linking studies, led the data analysis for generation of figures as well as assembly of the manuscript with D.E.I. D.D., M.R.S. and A.P. were responsible for DM-PBPK/PD model development and data analysis working closely with A.H., B.M.M., R.P.-B. and R.N. A.H., B.M.M., M.C., T.H. and S.S.F.J. planned and performed biological experiments with the help of R.N., Y.M., B.S., A.S.P. and S.J.-F., all under the supervision of O.L., A.C., R.N., R.P.-B., K.K.P. and D.E.I. R.N., B.C., K.K.P. and D.E.I. were responsible for chip development and fabrication. M.I., S.M, A.D. and R.N. were responsible for software and hardware engineering and operation of the linking studies. R.P.-B., R.N., K.K.P. and D.E.I. were responsible for overseeing the entire effort, including preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.E.I. is a founder and holds equity in Emulate, and chairs its scientific advisory board. K.K.P. is a consultant to Emulate. S.S.F.J. is an employee of and holds equity in Emulate. M.C., A.H., D.E.I., M.I., O.L., B.M.M., Y.M., R.N., K.K.P., A.S.-P. and D.E.I. are listed as inventors on intellectual property licensed to Emulate. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–8, Figs. 1–15 and references.
Rights and permissions
About this article
Cite this article
Herland, A., Maoz, B.M., Das, D. et al. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat Biomed Eng 4, 421–436 (2020). https://doi.org/10.1038/s41551-019-0498-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0498-9
This article is cited by
-
Microfluidic high-throughput 3D cell culture
Nature Reviews Bioengineering (2024)
-
A Scoping Review on the Advent of Microfluidic Devices in Dentistry
Current Oral Health Reports (2024)
-
Measuring and modelling tumour heterogeneity across scales
Nature Reviews Bioengineering (2023)
-
Human disease models in drug development
Nature Reviews Bioengineering (2023)
-
A high-throughput, 28-day, microfluidic model of gingival tissue inflammation and recovery
Communications Biology (2023)