Abstract
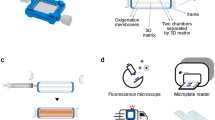
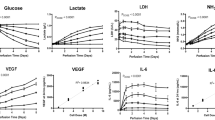
The inaccessibility of living bone marrow (BM) hampers the study of its pathophysiology under myelotoxic stress induced by drugs, radiation or genetic mutations. Here, we show that a vascularized human BM-on-a-chip (BM chip) supports the differentiation and maturation of multiple blood cell lineages over 4 weeks while improving CD34+ cell maintenance, and that it recapitulates aspects of BM injury, including myeloerythroid toxicity after clinically relevant exposures to chemotherapeutic drugs and ionizing radiation, as well as BM recovery after drug-induced myelosuppression. The chip comprises a fluidic channel filled with a fibrin gel in which CD34+ cells and BM-derived stromal cells are co-cultured, a parallel channel lined by human vascular endothelium and perfused with culture medium, and a porous membrane separating the two channels. We also show that BM chips containing cells from patients with the rare genetic disorder Shwachman–Diamond syndrome reproduced key haematopoietic defects and led to the discovery of a neutrophil maturation abnormality. As an in vitro model of haematopoietic dysfunction, the BM chip may serve as a human-specific alternative to animal testing for the study of BM pathophysiology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All of the data supporting the results in this study are available within the Article and its Supplementary Information. The broad range of raw datasets acquired and analysed (or any subsets of it), which for reuse would require contextual metadata, are available from the corresponding author on reasonable request.
Change history
12 February 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41551-020-0529-6
References
Doulatov, S., Notta, F., Laurenti, E. & Dick, J. E. Hematopoiesis: a human perspective. Cell Stem Cell 10, 120–136 (2012).
Eaves, C. J. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood 125, 2605–2613 (2015).
Wognum, B., Yuan, N., Lai, B. & Miller, C. L. Colony forming cell assays for human hematopoietic progenitor cells. Methods Mol. Biol. 946, 267–283 (2013).
Dexter, T. M., Allen, T. D. & Lajtha, L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J. Cell Physiol. 91, 335–344 (1977).
Sieber, S. et al. Bone marrow-on-a-chip: long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J. Tissue Eng. Regen. Med. 12, 479–489 (2018).
Thon, J. N. et al. Platelet bioreactor-on-a-chip. Blood 124, 1857–1867 (2014).
Di Maggio, N. et al. Toward modeling the bone marrow niche using scaffold-based 3D culture systems. Biomaterials 32, 321–329 (2011).
Rodling, L. et al. 3D models of the hematopoietic stem cell niche under steady-state and active conditions. Sci. Rep. 7, 4625 (2017).
Bourgine, P. E. et al. In vitro biomimetic engineering of a human hematopoietic niche with functional properties. Proc. Natl Acad. Sci. USA 115, E5688–E5695 (2018).
Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014).
Boitano, A. E. et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329, 1345–1348 (2010).
Fares, I. et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science 345, 1509–1512 (2014).
Ferreira, M. S. et al. Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials 33, 6987–6997 (2012).
Lee, H. Y. et al. PPAR-α and glucocorticoid receptor synergize to promote erythroid progenitor self-renewal. Nature 522, 474–477 (2015).
Pineault, N. & Abu-Khader, A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp. Hematol. 43, 498–513 (2015).
Pessina, A. et al. Application of the CFU-GM assay to predict acute drug-induced neutropenia: an international blind trial to validate a prediction model for the maximum tolerated dose (MTD) of myelosuppressive xenobiotics. Toxicol. Sci. 75, 355–367 (2003).
Boulais, P. E. & Frenette, P. S. Making sense of hematopoietic stem cell niches. Blood 125, 2621–2629 (2015).
Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010).
Sacchetti, B. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007).
Rafii, S., Butler, J. M. & Ding, B. S. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325 (2016).
Hassell, B. A. et al. Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro. Cell Rep. 21, 508–516 (2017).
Huh, D. et al. Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010).
Dorrell, C., Gan, O. I., Pereira, D. S., Hawley, R. G. & Dick, J. E. Expansion of human cord blood CD34+CD38− cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood 95, 102–110 (2000).
Von Laer, D. et al. Loss of CD38 antigen on CD34+CD38+ cells during short-term culture. Leukemia 14, 947–948 (2000).
Danet, G. H., Lee, H. W., Luongo, J. L., Simon, M. C. & Bonnet, D. A. Dissociation between stem cell phenotype and NOD/SCID repopulating activity in human peripheral blood CD34+ cells after ex vivo expansion. Exp. Hematol. 29, 1465–1473 (2001).
Csaszar, E. et al. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell 10, 218–229 (2012).
Jalili-Firoozinezhad, S. et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 3, 520–531 (2019).
Longley, D. B., Harkin, D. P. & Johnston, P. G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer 3, 330–338 (2003).
Lee, J. J., Beumer, J. H. & Chu, E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother. Pharm. 78, 447–464 (2016).
Saif, M. W., Choma, A., Salamone, S. J. & Chu, E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J. Natl Cancer Inst. 101, 1543–1552 (2009).
Santini, J. et al. 5-FU therapeutic monitoring with dose adjustment leads to an improved therapeutic index in head and neck cancer. Br. J. Cancer 59, 287–290 (1989).
Trump, D. L. et al. Pharmacokinetic and pharmacodynamic analysis of fluorouracil during 72-hour continuous infusion with and without dipyridamole. J. Clin. Oncol. 9, 2027–2035 (1991).
Malerba, I., Casati, S., Diodovich, C., Parent-Massin, D. & Gribaldo, L. Inhibition of CFU-E/BFU-E and CFU-GM colony growth by cyclophosphamide, 5-fluorouracil and taxol: development of a high-throughput in vitro method. Toxicol. Vitr. 18, 293–300 (2004).
Carmena, M., Wheelock, M., Funabiki, H. & Earnshaw, W. C. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 13, 789–803 (2012).
Boss, D. S. et al. Clinical evaluation of AZD1152, an i.v. inhibitor of Aurora B kinase, in patients with solid malignant tumors. Ann. Oncol. 22, 431–437 (2011).
Kantarjian, H. M. et al. Stage I of a phase 2 study assessing the efficacy, safety, and tolerability of barasertib (AZD1152) versus low-dose cytosine arabinoside in elderly patients with acute myeloid leukemia. Cancer 119, 2611–2619 (2013).
Schwartz, G. K. et al. Phase I study of barasertib (AZD1152), a selective inhibitor of Aurora B kinase, in patients with advanced solid tumors. Invest. New Drugs 31, 370–380 (2013).
Yang, J. et al. AZD1152, a novel and selective Aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood 110, 2034–2040 (2007).
Waselenko, J. K. et al. Medical management of the acute radiation syndrome: recommendations of the strategic national stockpile radiation working group. Ann. Intern. Med. 140, 1037–1051 (2004).
Singh, V. K. & Seed, T. M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int. J. Radiat. Biol. 93, 851–869 (2017).
Dror, Y. et al. Draft consensus guidelines for diagnosis and treatment of Shwachman–Diamond syndrome. Ann. NY Acad. Sci. 1242, 40–55 (2011).
Myers, K. C., Davies, S. M. & Shimamura, A. Clinical and molecular pathophysiology of Shwachman–Diamond syndrome: an update. Hematol. Oncol. Clin. North Am. 27, 117–128 (2013).
Bezzerri, V. & Cipolli, M. Shwachman–Diamond syndrome: molecular mechanisms and current perspectives. Mol. Diagnosis Ther. 23, 281–290 (2019).
Myers, K. C. et al. Variable clinical presentation of Shwachman–Diamond syndrome: update from the North American Shwachman–Diamond syndrome registry. J. Pediatr. 164, 866–870 (2014).
Finch, A. J. et al. Uncoupling of GTP hydrolysis from eIF6 release on the ribosome causes Shwachman–Diamond syndrome. Genes Dev. 25, 917–929 (2011).
Raaijmakers, M. H. et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464, 852–857 (2010).
Rawls, A. S., Gregory, A. D., Woloszynek, J. R., Liu, F. & Link, D. C. Lentiviral-mediated RNAi inhibition of Sbds in murine hematopoietic progenitors impairs their hematopoietic potential. Blood 110, 2414–2422 (2007).
Zambetti, N. A. et al. Deficiency of the ribosome biogenesis gene Sbds in hematopoietic stem and progenitor cells causes neutropenia in mice by attenuating lineage progression in myelocytes. Haematologica 100, 1285–1293 (2015).
Zhang, S., Shi, M., Hui, C. C. & Rommens, J. M. Loss of the mouse ortholog of the Shwachman–Diamond syndrome gene (Sbds) results in early embryonic lethality. Mol. Cell Biol. 26, 6656–6663 (2006).
Dror, Y. & Freedman, M. H. Shwachman–Diamond syndrome: an inherited preleukemic bone marrow failure disorder with aberrant hematopoietic progenitors and faulty marrow microenvironment. Blood 94, 3048–3054 (1999).
Kuijpers, T. W. et al. Hematologic abnormalities in Shwachman Diamond syndrome: lack of genotype–phenotype relationship. Blood 106, 356–361 (2005).
Walasek, M. A., van Os, R. & de Haan, G. Hematopoietic stem cell expansion: challenges and opportunities. Ann. NY Acad. Sci. 1266, 138–150 (2012).
Lis, R. et al. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature 545, 439–445 (2017).
Sugimura, R. et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature 545, 432–438 (2017).
Kohn, L. A. et al. Lymphoid priming in human bone marrow begins before expression of CD10 with upregulation of l-selectin. Nat. Immunol. 13, 963–971 (2012).
Rawlings, D. J., Quan, S. G., Kato, R. M. & Witte, O. N. Long-term culture system for selective growth of human B-cell progenitors. Proc. Natl Acad. Sci. USA 92, 1570–1574 (1995).
Harrison, J. S., Rameshwar, P., Chang, V. & Bandari, P. Oxygen saturation in the bone marrow of healthy volunteers. Blood 99, 394 (2002).
Nombela-Arrieta, C. et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat. Cell Biol. 15, 533–543 (2013).
Spencer, J. A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014).
Broxmeyer, H. E., O’Leary, H. A., Huang, X. & Mantel, C. The importance of hypoxia and extra physiologic oxygen shock/stress for collection and processing of stem and progenitor cells to understand true physiology/pathology of these cells ex vivo. Curr. Opin. Hematol. 22, 273–278 (2015).
Andre, V. et al. Mesenchymal stem cells from Shwachman–Diamond syndrome patients display normal functions and do not contribute to hematological defects. Blood Cancer J. 2, e94 (2012).
Dror, Y. et al. Immune function in patients with Shwachman–Diamond syndrome. Br. J. Haematol. 114, 712–717 (2001).
Orelio, C. & Kuijpers, T. W. Shwachman–Diamond syndrome neutrophils have altered chemoattractant-induced F-actin polymerization and polarization characteristics. Haematologica 94, 409–413 (2009).
Novak, R. et al. Scalable fabrication of stretchable, dual channel, microfluidic organ chips. J. Vis. Exp. 140, e58151 (2018).
Keizer, R. J., Zandvliet, A. S., Beijnen, J. H., Schellens, J. H. & Huitema, A. D. Two-stage model-based design of cancer phase I dose escalation trials: evaluation using the phase I program of barasertib (AZD1152). Invest. New Drugs 30, 1519–1530 (2012).
Acknowledgements
This research was sponsored by funding from: the US Food and Drug Administration (grants HHSF223201310079C and 75F40119C10098), the Defense Advanced Research Projects Agency (under Cooperative Agreement Number W911NF-12-2-0036), AstraZeneca and the Wyss Institute for Biologically Inspired Engineering (to D.E.I.); the US National Institutes of Health (R24 DK099808 and 5U01HL134812 to A.S., R01 DK102165 to C.D.N. and training grant 5T32CA009216-37 to D.B.C.); and the Department of Defense (W81XWH-14-1-0124 to C.D.N.). Additional funding was provided by the Dana-Farber Cancer Center Claudia Adams Barr Award (to C.E.J.) and the EPSRC Centre for Innovative Manufacturing in Regenerative Medicine (to A.R.). The authors thank S. Sweeney for helpful discussions, P. Machado and J. Caramanica for machining expertise, and M. DeLelys, R. Mathews, J. Houston, J. Patel, D. Kingman, A. Shay, J. Graham, S. Chung, T. Spitzer and F. Preffer at the Massachusetts General Hospital, as well as M. Fleming and M. Armant at Boston Children’s Hospital for invaluable help in relation to working with patient data and samples.
Author information
Authors and Affiliations
Contributions
D.B.C. and V.F. participated in the design and performance of all experiments and analysed the data, alongside D.E.I., who also supervised all of the work. Y.M. helped design and perform the experiments. R.D., P.P.-D., D.F., A.M. and L.E. helped to design experiments relating to drug testing, performed the mass spectrometry and pharmacokinetics modelling, analysed the data and helped to write the manuscript. O.V.B. and C.E.J. helped to design, perform and interpret SDS-related studies, with input from and supervision of A.S. and C.D.N. K.C.M. and O.K.W. provided access to patient data and material for SDS-related studies. L.S.M.T. and A.R. helped to conceive the BM chip design and performed the experiments. A.J. helped to perform the radiation-related studies. B.A.F. and L.R.O. helped to analyse the data and revise the manuscript. E.C., C.F.N., Y.C. and S.C. fabricated and participated in the design of the BM chip with input from and supervision of R.N. and D.E.I. E.C., S.J.-F. and S.C. helped to perform the oxygen studies. R.P.H. provided scientific supervision, as well as access to patient material. O.L. and R.P.-B. helped to design the experiments and interpret the data, and supervised all of the work. D.B.C., V.F. and D.E.I. prepared the manuscript, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
D.E.I. is a founder, and holds equity in, Emulate, Inc., and chairs its scientific advisory board. D.B.C., V.F., Y.M., L.S.M.T., O.L., R.N. and D.E.I. are co-inventors on a patent application describing the BM chip. R.D., P.P.-D., D.F., A.M. and L.E. are employed by AstraZeneca, which is developing AZD2811. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Table 1 and references.
Rights and permissions
About this article
Cite this article
Chou, D.B., Frismantas, V., Milton, Y. et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat Biomed Eng 4, 394–406 (2020). https://doi.org/10.1038/s41551-019-0495-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0495-z
This article is cited by
-
Microfluidic high-throughput 3D cell culture
Nature Reviews Bioengineering (2024)
-
Generating human bone marrow organoids for disease modeling and drug discovery
Nature Protocols (2024)
-
Advances and challenges in organ-on-chip technology: toward mimicking human physiology and disease in vitro
Medical & Biological Engineering & Computing (2024)
-
Generation of complex bone marrow organoids from human induced pluripotent stem cells
Nature Methods (2024)
-
Human disease models in drug development
Nature Reviews Bioengineering (2023)