Abstract
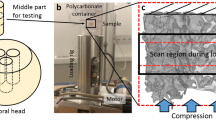
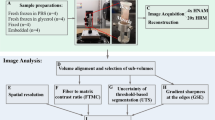
Imaging techniques for quantifying changes in the hierarchical structure of deforming joints are constrained by destructive sample treatments, sample-size restrictions and lengthy scan times. Here, we report the use of fast low-dose pink-beam synchrotron X-ray tomography in combination with mechanical loading at nanometric precision for in situ imaging, at resolutions below 100 nm, of the mechanical strain in intact untreated joints under physiologically realistic conditions. We show that in young, older and osteoarthritic mice, hierarchical changes in tissue structure and mechanical behaviour can be simultaneously visualized, and that the tissue structure at the cellular level correlates with the mechanical performance of the whole joint. We also use the tomographic approach to study the colocalization of tissue strains to specific chondrocyte lacunar organizations within intact loaded joints and to explore the role of calcified-cartilage stiffness on the biomechanics of healthy and pathological joints.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Representative samples of research data from the experiments and of the data for the figures in the manuscript are provided in the Supplementary Information. The full data, of considerable size, are available from the corresponding authors on reasonable request.
Code availability
The custom DVC code used in this study is available at https://zenodo.org/record/3228175#.XZdBRkZKguE.
References
Denk, W. & Horstmann, H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2, e329 (2004).
Georgiadis, M., Mueller, R. & Schneider, P. Techniques to assess bone ultrastructure organization: orientation and arrangement of mineralized collagen fibrils. J. R. Soc. Interface 13, 20160088 (2016).
Pabisch, S., Wagermaier, W., Zander, T., Li, C. & Fratzl, P. in Methods in Enzymology Vol. 532 (ed. De Yoreo, J.) 391–413 (Elsevier, 2013).
Zhu, F.-Y. et al. 3D nanostructure reconstruction based on the SEM imaging principle, and applications. Nanotechnology 25, 185705 (2014).
Gupta, H. S. et al. Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc. Natl Acad. Sci. USA 103, 17741–17746 (2006).
Tadano, S., Giri, B., Sato, T., Fujisaki, K. & Todoh, M. Estimating nanoscale deformation in bone by X-ray diffraction imaging method. J. Biomech. 41, 945–952 (2008).
Orgel, J. P., Irving, T. C., Miller, A. & Wess, T. J. Microfibrillar structure of type I collagen in situ. Proc. Natl Acad. Sci. USA 103, 9001–9005 (2006).
Gautieri, A., Vesentini, S., Redaelli, A. & Buehler, M. J. Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Lett. 11, 757–766 (2011).
Dierolf, M. et al. Ptychographic X-ray computed tomography at the nanoscale. Nature 467, 436–439 (2010).
Giannuzzi, L. A., Phifer, D., Giannuzzi, N. J. & Capuano, M. J. Two-dimensional and 3-dimensional analysis of bone/dental implant interfaces with the use of focused ion beam and electron microscopy. J. Oral Maxillofac. Surg. 65, 737–747 (2007).
Schneider, P., Meier, M., Wepf, R. & Müller, R. Serial FIB/SEM imaging for quantitative 3D assessment of the osteocyte lacuno-canalicular network. Bone 49, 304–311 (2011).
Boyde, A. & Jones, S. J. Scanning electron microscopy of bone: instrument, specimen, and issues. Microsc. Res. Tech. 33, 92–120 (1996).
Song, M. J., Dean, D. & Tate, M. L. K. In situ spatiotemporal mapping of flow fields around seeded stem cells at the subcellular length scale. PLoS ONE 5, e12796 (2010).
Roeder, B. A., Kokini, K., Robinson, J. P. & Voytik-Harbin, S. L. Local, three-dimensional strain measurements within largely deformed extracellular matrix constructs. J. Biomech. Eng. 126, 699–708 (2004).
Sztefek, P. et al. Using digital image correlation to determine bone surface strains during loading and after adaptation of the mouse tibia. J. Biomech. 43, 599–605 (2010).
Hoc, T. et al. Effect of microstructure on the mechanical properties of Haversian cortical bone. Bone 38, 466–474 (2006).
Bay, B. K. Texture correlation: a method for the measurement of detailed strain distributions within trabecular bone. J. Orthop. Res. 13, 258–267 (1995).
Nicolella, D. P., Moravits, D. E., Gale, A. M., Bonewald, L. F. & Lankford, J. Osteocyte lacunae tissue strain in cortical bone. J. Biomech. 39, 1735–1743 (2006).
Katsamenis, O. L., Chong, H. M., Andriotis, O. G. & Thurner, P. J. Load-bearing in cortical bone microstructure: Selective stiffening and heterogeneous strain distribution at the lamellar level. J. Mech. Behav. Biomed. Mater. 17, 152–165 (2013).
Tai, K., Dao, M., Suresh, S., Palazoglu, A. & Ortiz, C. Nanoscale heterogeneity promotes energy dissipation in bone. Nat. Mater. 6, 454–462 (2007).
Hassenkam, T. et al. High-resolution AFM imaging of intact and fractured trabecular bone. Bone 35, 4–10 (2004).
Thurner, P. J. et al. in Modern Research and Educational Topics in Microscopy (eds Méndez-Vilas, A. D. & Díaz, J.) 37–48 (Formatex, 2007).
Pan, B. & Wang, B. A flexible and accurate digital volume correlation method applicable to high-resolution volumetric images. Meas. Sci. Technol. 28, 105007 (2017).
Hussein, A. I., Barbone, P. E. & Morgan, E. F. Digital volume correlation for study of the mechanics of whole bones. Procedia IUTAM 4, 116–125 (2012).
Bay, B. K., Smith, T. S., Fyhrie, D. P. & Saad, M. Digital volume correlation: three-dimensional strain mapping using X-ray tomography. Exp. Mech. 39, 217–226 (1999).
Roberts, B. C., Perilli, E. & Reynolds, K. J. Application of the digital volume correlation technique for the measurement of displacement and strain fields in bone: a literature review. J. Biomech. 47, 923–934 (2014).
Barth, H. D., Launey, M. E., MacDowell, A. A., Ager, J. W. III & Ritchie, R. O. On the effect of X-ray irradiation on the deformation and fracture behavior of human cortical bone. Bone 46, 1475–1485 (2010).
Currey, J. D. et al. Effects of ionizing radiation on the mechanical properties of human bone. J. Orthop. Res. 15, 111–117 (1997).
Staines, K. A., Poulet, B., Wentworth, D. N. & Pitsillides, A. A. The STR/ort mouse model of spontaneous osteoarthritis–an update. Osteoarthr. Cartilage 25, 802–808 (2017).
De Fanis, A., Pešić, Z., Wagner, U. & Rau, C. Fast X-ray imaging at beamline I13L at Diamond Light Source. J. Phys. Conf. Ser. 425, 192014 (2013).
Karagadde, S. et al. Transgranular liquation cracking of grains in the semi-solid state. Nat. Commun. 6, 8300 (2015).
Kareh, K., Lee, P., Atwood, R., Connolley, T. & Gourlay, C. Revealing the micromechanisms behind semi-solid metal deformation with time-resolved X-ray tomography. Nat. Commun. 5, 4464 (2014).
Bay, B. K. Methods and applications of digital volume correlation. J. Strain Anal. Eng. Des. 43, 745–760 (2008).
Goldring, M. B. & Goldring, S. R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 1192, 230–237 (2010).
Lories, R. J. & Luyten, F. P. The bone–cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 7, 43–49 (2011).
Müller, R. Hierarchical microimaging of bone structure and function. Nat. Rev. Rheumatol. 5, 373–381 (2009).
Yamada, S., Tadano, S. & Fujisaki, K. Residual stress distribution in rabbit limb bones. J. Biomech. 44, 1285–1290 (2011).
Gupta, H. S. et al. Nanoscale deformation mechanisms in bone. Nano Lett. 5, 2108–2111 (2005).
Campbell, S. E., Ferguson, V. L. & Hurley, D. C. Nanomechanical mapping of the osteochondral interface with contact resonance force microscopy and nanoindentation. Acta Biomaterialia 8, 4389–4396 (2012).
Mente, P. & Lewis, J. L. Elastic modulus of calcified cartilage is an order of magnitude less than that of subchondral bone. J. Orthop. Res. 12, 637–647 (1994).
Hargrave-Thomas, E., van Sloun, F., Dickinson, M., Broom, N. & Thambyah, A. Multi-scalar mechanical testing of the calcified cartilage and subchondral bone comparing healthy vs early degenerative states. Osteoarthr. Cartilage 23, 1755–1762 (2015).
Doube, M., Firth, E. & Boyde, A. Variations in articular calcified cartilage by site and exercise in the 18-month-old equine distal metacarpal condyle. Osteoarthr. Cartilage 15, 1283–1292 (2007).
Day, J. et al. Adaptation of subchondral bone in osteoarthritis. Biorheology 41, 359–368 (2004).
Li, B. & Aspden, R. M. Mechanical and material properties of the subchondral bone plate from the femoral head of patients with osteoarthritis or osteoporosis. Ann. Rheum. Dis. 56, 247–254 (1997).
Li, B. & Aspden, R. M. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J. Bone Miner. Res. 12, 641–651 (1997).
Jaiprakash, A. et al. Phenotypic characterization of osteoarthritic osteocytes from the sclerotic zones: a possible pathological role in subchondral bone sclerosis. Int. J. Biol. Sci. 8, 406–417 (2012).
Couchourel, D. et al. Altered mineralization of human osteoarthritic osteoblasts is attributable to abnormal type I collagen production. Arthritis Rheum. 60, 1438–1450 (2009).
Poulet, B. et al. Intermittent applied mechanical loading induces subchondral bone thickening that may be intensified locally by contiguous articular cartilage lesions. Osteoarthr. Cartilage 23, 940–948 (2015).
Van Ruijven, L., Mulder, L. & Van Eijden, T. Variations in mineralization affect the stress and strain distributions in cortical and trabecular bone. J. Biomech. 40, 1211–1218 (2007).
Mori, S., Harruff, R. & Burr, D. Microcracks in articular calcified cartilage of human femoral heads. Arch. Pathol. Lab. Med. 117, 196–198 (1993).
Pan, J. et al. Elevated cross-talk between subchondral bone and cartilage in osteoarthritic joints. Bone 51, 212–217 (2012).
Suri, S. & Walsh, D. A. Osteochondral alterations in osteoarthritis. Bone 51, 204–211 (2012).
Pouran, B. et al. Solute transport at the interface of cartilage and subchondral bone plate: effect of micro-architecture. J. Biomech. 52, 148–154 (2017).
Muir, P. et al. Role of endochondral ossification of articular cartilage and functional adaptation of the subchondral plate in the development of fatigue microcracking of joints. Bone 38, 342–349 (2006).
Laverty, S., Lacourt, M., Gao, C., Henderson, J. & Boyde, A. High density infill in cracks and protrusions from the articular calcified cartilage in osteoarthritis in standardbred horse carpal bones. Int. J. Mol. Sci. 16, 9600–9611 (2015).
Boyde, A. et al. On fragmenting, densely mineralised acellular protrusions into articular cartilage and their possible role in osteoarthritis. J. Anat. 225, 436–446 (2014).
Turley, S. M., Thambyah, A., Riggs, C. M., Firth, E. C. & Broom, N. D. Microstructural changes in cartilage and bone related to repetitive overloading in an equine athlete model. J. Anat. 224, 647–658 (2014).
Boyde, A. et al. Cartilage damage involving extrusion of mineralisable matrix from the articular calcified cartilage and subchondral bone. Eur. Cells Mater. 21, 470–478 (2011).
Boyde, A. The real response of bone to exercise. J. Anat. 203, 173–189 (2003).
Comhaire, F. H. & Snaps, F. Comparison of two canine registry databases on the prevalence of hip dysplasia by breed and the relationship of dysplasia with body weight and height. Am. J. Vet. Res. 69, 330–333 (2008).
Staines, K., Pollard, A., McGonnell, I., Farquharson, C. & Pitsillides, A. Cartilage to bone transitions in health and disease. J. Endocrinol. 219, R1–R12 (2013).
Staines, K. et al. Endochondral growth defect and deployment of transient chondrocyte behaviors underlie osteoarthritis onset in a natural murine model. Arthritis Rheumatol. 68, 880–891 (2016).
Pitsillides, A. A. & Beier, F. Cartilage biology in osteoarthritis—lessons from developmental biology. Nat. Rev. Rheumatology 7, 654–663 (2011).
Puncreobutr, C., Lee, P., Hamilton, R. & Phillion, A. Quantitative 3D characterization of solidification structure and defect evolution in Al alloys. JOM 64, 89–95 (2012).
Maksimcuka, J. et al. X-ray tomographic imaging of tensile deformation modes of electrospun biodegradable polyester fibers. Front. Mater. 4, 43 (2017).
Poulet, B., Hamilton, R. W., Shefelbine, S. & Pitsillides, A. A. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 63, 137–147 (2011).
Nazarian, A., Stauber, M., Zurakowski, D., Snyder, B. D. & Müller, R. The interaction of microstructure and volume fraction in predicting failure in cancellous bone. Bone 39, 1196–1202 (2006).
Rau, C., Wagner, U., Pešić, Z. & De Fanis, A. Coherent imaging at the Diamond beamline I13. Physica Status Solidi A 208, 2522–2525 (2011).
Pešić, Z., De Fanis, A., Wagner, U. & Rau, C. Experimental stations at I13 beamline at Diamond Light Source. J. Phys. Conf. Ser. 425, 182003 (2013).
Christen, D. et al. Deformable image registration and 3D strain mapping for the quantitative assessment of cortical bone microdamage. J. Mech. Behav. Biomed. Mater. 8, 184–193 (2012).
Voide, R. et al. Time-lapsed assessment of microcrack initiation and propagation in murine cortical bone at submicrometer resolution. Bone 45, 164–173 (2009).
Pacureanu, A., Langer, M., Boller, E., Tafforeau, P. & Peyrin, F. Nanoscale imaging of the bone cell network with synchrotron X‐ray tomography: optimization of acquisition setup. Med. Phys. 39, 2229–2238 (2012).
Basham, M. et al. Data analysis workbench (DAWN). J. Synchrotron Radiat. 22, 853–858 (2015).
Titarenko, V. Analytical formula for two-dimensional ring artefact suppression. J. Synchrotron Radiat. 23, 1447–1461 (2016).
Madi, K. et al. Computation of full-field displacements in a scaffold implant using digital volume correlation and finite element analysis. Med. Eng. Phys. 35, 1298–1312 (2013).
Abd-Elmoniem, K. Z., Stuber, M. & Prince, J. L. Direct three-dimensional myocardial strain tensor quantification and tracking using zHARP. Med. Image Anal. 12, 778–786 (2008).
De Souza, R. L. et al. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 37, 810–818 (2005).
Acknowledgements
We are grateful to R. Mason (Imperial College London) for providing our original STR/Ort mice and for advice on their use. We thank L. Courtois, S. V. Boxel, C. Disney, G. Poologasundarampillai, J. He, D. Eastwood, K. Wanelik, U. Wagner and J. Thompson for their help during the beamtimes. We acknowledge the Engineering and Physical Sciences Research Council (grants EP/I02249X/1 and EP/M009688/1), Arthritis Research UK (grant 18768) and the MRC (grant MR/R025673/1). Facilities and research support were provided by the Diamond-Manchester Branchline (I13-2) at Diamond Light Source (Beamtimes MT13237-1, MT11076-1 and MT5003-1) and the Research Complex at Harwell.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: P.D.L., A.A.P., K.M., K.A.S. and B.K.B. Acquisition of data: K.M., B.K.B., H.G., B.J., K.A.S. and A.J.B. Interpretation of data, revision of the manuscript, final approval and agreement to be accountable for all aspects of the work: all authors. Drafting of the manuscript: K.A.S., K.M., B.K.B., A.A.P. and P.D.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures, Supplementary Tables and Supplementary References.
Rights and permissions
About this article
Cite this article
Madi, K., Staines, K.A., Bay, B.K. et al. In situ characterization of nanoscale strains in loaded whole joints via synchrotron X-ray tomography. Nat Biomed Eng 4, 343–354 (2020). https://doi.org/10.1038/s41551-019-0477-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0477-1
This article is cited by
-
Preparation of large biological samples for high-resolution, hierarchical, synchrotron phase-contrast tomography with multimodal imaging compatibility
Nature Protocols (2023)
-
Imaging Cu2O nanocube hollowing in solution by quantitative in situ X-ray ptychography
Nature Communications (2022)
-
Anatomical Distribution of Ochronotic Pigment in Alkaptonuric Mice is Associated with Calcified Cartilage Chondrocytes at Osteochondral Interfaces
Calcified Tissue International (2021)
-
Resolving nanoscale strains in whole joints
Nature Biomedical Engineering (2020)