Abstract
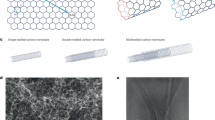
Mechanical mismatches between implanted electronics and biological tissues can lead to inaccurate readings and long-term tissue damage. Here, we show that functionalized multi-walled carbon nanotubes twisted into helical fibre bundles that mimic the hierarchical structure of muscle can monitor multiple disease biomarkers in vivo. The flexible fibre bundles are injectable, have a low bending stiffness and display ultralow stress under compression. As proof-of-concept evidence of the sensing capabilities of these fibre bundles, we show that the fibre bundles enable the spatially resolved and real-time monitoring of H2O2 when implanted in tumours in mice, and that they can be integrated with a wireless transmission system on an adhesive skin patch to monitor calcium ions and glucose in the venous blood of cats for 28 d. The versatility of the helical fibre bundles as chemically functionalized electrochemical sensors makes them suitable for multiple sensing applications in biomedicine and healthcare.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets that were generated during this study are available from the corresponding authors on reasonable request.
References
Clausen, J. Man, machine and in between. Nature 457, 1080–1081 (2009).
Yoshida Kozai, T. D. et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 11, 1065–1073 (2012).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Yun, S. H. & Kwok, S. J. J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 1, 0008 (2017).
Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
An, D. et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc. Natl Acad. Sci. USA 115, E263–E272 (2018).
Vermesh, O. et al. An intravascular magnetic wire for the high-throughput retrieval of circulating tumour cells in vivo. Nat. Biomed. Eng. 2, 696–705 (2018).
Feiner, R. & Dvir, T. Tissue–electronics interfaces: from implantable devices to engineered tissues. Nat. Rev. Mater. 3, 17076 (2017).
Yu, X. et al. Needle-shaped ultrathin piezoelectric microsystem for guided tissue targeting via mechanical sensing. Nat. Biomed. Eng. 2, 165–172 (2018).
Lacour, S. P., Courtine, G. & Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 1, 16063 (2016).
Chen, R., Canales, A. & Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2, 16093 (2017).
Hong, G. & Lieber, C. M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 20, 330–345 (2019).
Yang, X. et al. Bioinspired neuron-like electronics. Nat. Mater. 18, 510–517 (2019).
Ling, S., Kaplan, D. L. & Buehler, M. J. Nanofibrils in nature and materials engineering. Nat. Rev. Mater. 3, 18016 (2018).
Buehler, M. J. Nature designs tough collagen: explaining the nanostructure of collagen fibrils. Proc. Natl Acad. Sci. USA 103, 12285–12290 (2006).
Hang, F. & Barber, A. H. Nano-mechanical properties of individual mineralized collagen fibrils from bone tissue. J. R. Soc. Interface 8, 500–505 (2011).
Depalle, B., Qin, Z., Shefelbine, S. J. & Buehler, M. J. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J. Mech. Behav. Biomed. Mater. 52, 1–13 (2015).
Dai, X., Hong, G., Gao, T. & Lieber, C. M. Mesh nanoelectronics: seamless integration of electronics with tissues. Acc. Chem. Res. 51, 309–318 (2018).
Darnell, M. & Mooney, D. J. Leveraging advances in biology to design biomaterials. Nat. Mater. 16, 1178–1184 (2017).
Baker, B. M. et al. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 14, 1262–1268 (2015).
Waldert, S. Invasive vs. non-invasive neuronal signals for brain–machine interfaces: will one prevail? Front. Neurosci. 10, 295 (2016).
Vitale, F. et al. Fluidic microactuation of flexible electrodes for neural recording. Nano Lett. 18, 326–335 (2018).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015).
Close, R. I. Dynamic properties of mammalian skeletal muscles. Physiol. Rev. 52, 129–197 (1972).
Alonso, J. L. & Goldmann, W. H. Feeling the forces: atomic force microscopy in cell biology. Life Sci. 72, 2553–2560 (2003).
Liu, Y., Zhao, Y., Sun, B. & Chen, C. Understanding the toxicity of carbon nanotubes. Acc. Chem. Res. 46, 702–713 (2013).
Jhunjhunwala, S. et al. Neutrophil responses to sterile implant materials. PLoS ONE 10, e0137550 (2015).
Gurtner, G. C., Werner, S., Barrandon, Y. & Longaker, M. T. Wound repair and regeneration. Nature 453, 314–321 (2008).
Yang, Z. et al. Recent advancement of nanostructured carbon for energy applications. Chem. Rev. 115, 5159–5223 (2015).
D’Amico, A. V., Chen, M.-H., Roehl, K. A. & Catalona, W. J. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N. Engl. J. Med. 351, 125–135 (2004).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Zhu, S., Nih, L., Carmichael, S. T., Lu, Y. & Segura, T. Enzyme-responsive delivery of multiple proteins with spatiotemporal control. Adv. Mater. 27, 3620–3625 (2015).
Oxley, T. J. et al. Minimally invasive endovascular stent-electrode array for high-fidelity, chronic recordings of cortical neural activity. Nat. Biotechnol. 34, 320–327 (2016).
Luo, Y., Liu, H., Rui, Q. & Tian, Y. Detection of extracellular H2O2 released from human liver cancer cells based on TiO2 nanoneedles with enhanced electron transfer of cytochrome c. Anal. Chem. 81, 3035–3041 (2009).
Rodenhizer, D. et al. A three-dimensional engineered tumour for spatial snapshot analysis of cell metabolism and phenotype in hypoxic gradients. Nat. Mater. 15, 227–234 (2016).
Chen, Q. et al. H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay. Proc. Natl Acad. Sci. USA 114, 5343–5348 (2017).
Hong, G., Antaris, A. L. & Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
Lipani, L. et al. Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform. Nat. Nanotechnol. 13, 504–511 (2018).
Lee, H. et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 3, e1601314 (2017).
Tarlani, A. et al. New ZnO nanostructures as non-enzymatic glucose biosensors. Biosens. Bioelectron. 67, 601–607 (2015).
Tang, Z., Fu, Y. & Ma, Z. Bovine serum albumin as an effective sensitivity enhancer for peptide-based amperometric biosensor for ultrasensitive detection of prostate specific antigen. Biosens. Bioelectron. 94, 394–399 (2017).
Chen, P. et al. Hierarchically arranged helical fibre actuators driven by solvents and vapours. Nat. Nanotechnol. 10, 1077–1083 (2015).
He, X. Q. et al. Buckling analysis of multi-walled carbon nanotubes: a continuum model accounting for van der Waals interaction. J. Mech. Phys. Solids 53, 303–326 (2005).
Ru, C. Q. Effect of van der Waals forces on axial buckling of a double-walled carbon nanotube. J. Appl. Phys. 87, 7227–7231 (2000).
Natsuki, T. et al. Stability analysis of double-walled carbon nanotubes as AFM probes based on a continuum model. Carbon 49, 2532–2537 (2011).
Timesli, A. et al. Prediction of the critical buckling load of multi-walled carbon nanotubes under axial compression. C. R. Mec. 345, 158–168 (2017).
Khosravian, N. & Rafii-Tabar, H. Computational modelling of a non-viscous fluid flow in a multi-walled carbon nanotube modelled as a Timoshenko beam. Nanotechnology 19, 275703 (2008).
Sun, H. et al. Energy harvesting and storage in 1D devices. Nat. Rev. Mater. 2, 17023 (2017).
Tang, T. et al. Adhesion between single-walled carbon nanotubes. J. Appl. Phys. 97, 074304 (2005).
Huang, S. Q. et al. Pattern instability of a soft elastic thin film under van der Waals forces. Mech. Mater. 38, 88–99 (2006).
Israelachvili, J. N. Intermolecular and Surface Forces (Academic, 2011).
Acknowledgements
We thank A. L. Chun of Science Storylab for her suggestions and critique; F. Han from Fudan University for help with the wireless-transmitting system. This work was supported by MOST (grant number 2016YFA0203302), NSFC (grant numbers 21634003, 51573027, 51673043, 21604012, 21805044, 21875042, 11602058 and 11872150), STCSM (grant numbers 16JC1400702, 17QA1400400, 18QA1400700, 18QA1400800 and 19QA1400500), SHMEC (grant number 2017-01-07-00-07-E00062), SEDF (grant number 16CG01) and Yanchang Petroleum Group and China Postdoctoral Science Foundation (grant numbers 2017M610223 and 2018T110334). This work was also supported by Shanghai Municipal Science and Technology Major Project (2018SHZDZX01) and ZJLab (to H.Y.).
Author information
Authors and Affiliations
Contributions
X.S., F.X., H.Y. and H.P. conceived and designed the research project. L.W., Z.W. and S.X. performed the experiments. F.L. and Y.Y. performed numerical simulations. L.W., S.X. and Z.W. analysed the data. All of the authors discussed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures, tables, references and caption for Supplementary Video 1.
Supplementary Video 1
Wireless monitoring system integrated with MSFs on a living cat for the real-time transmission of data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Xie, S., Wang, Z. et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat Biomed Eng 4, 159–171 (2020). https://doi.org/10.1038/s41551-019-0462-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0462-8
This article is cited by
-
Design and fabrication of wearable electronic textiles using twisted fiber-based threads
Nature Protocols (2024)
-
A highly adsorptive electrochemical fiber sensor for real-time and accurate detection of intracranial nitric oxide
Science China Materials (2024)
-
All-in-one multifunctional and stretchable electrochemical fiber enables health-monitoring textile with trace sweat
Science China Materials (2024)
-
Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring
Nano-Micro Letters (2024)
-
Semi-implantable device based on multiplexed microfilament electrode cluster for continuous monitoring of physiological ions
Bio-Design and Manufacturing (2024)