Abstract
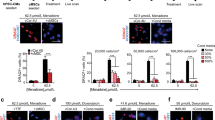
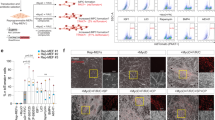
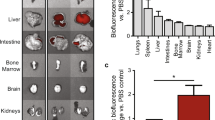
Cardiosphere-derived cells are therapeutic candidates with disease-modifying bioactivity, but their variable potency has complicated their clinical translation. Transcriptomic analyses of cardiosphere-derived cells from human donors have revealed that their therapeutic potency correlates with Wnt/β-catenin signalling and with β-catenin protein levels. Here, we show that skin fibroblasts engineered to overexpress β-catenin and the transcription factor Gata4 become immortal and therapeutically potent. Transplantation of the engineered fibroblasts into a mouse model of acute myocardial infarction led to improved cardiac function and mouse survival, and in the mdx mouse model of Duchenne muscular dystrophy, exosomes secreted by the engineered fibroblasts improved exercise capacity and reduced skeletal-muscle fibrosis. We also demonstrate that exosomes from high-potency cardiosphere-derived cells exhibit enhanced levels of miR-92a (a known potentiator of the Wnt/β-catenin pathway), and that they activate cardioprotective bone-morphogenetic-protein signalling in cardiomyocytes. Our findings show that the modulation of canonical Wnt signalling can turn therapeutically inert mammalian cells into immortal exosome factories for cell-free therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The authors declare that all data supporting the results in this study are available within the paper and its Supplementary Information.
References
Kreke, M., Smith, R. R., Marban, L. & Marban, E. Cardiospheres and cardiosphere-derived cells as therapeutic agents following myocardial infarction. Expert Rev. Cardiovasc. Ther. 10, 1185–1194 (2012).
Smith, R. R. et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115, 896–908 (2007).
Aminzadeh, M. A. et al. Exosome-mediated benefits of cell therapy in mouse and human models of duchenne muscular dystrophy. Stem Cell Rep. 10, 942–955 (2018).
Ashur, C. & Frishman, W. H. Cardiosphere-derived cells and ischemic heart failure. Cardiol. Rev. 26, 8–21 (2018).
Oh, H. Cell therapy trials in congenital heart disease. Circ. Res. 120, 1353–1366 (2017).
Chimenti, I. et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 106, 971–980 (2010).
Galvez, B. G. et al. Human cardiac mesoangioblasts isolated from hypertrophic cardiomyopathies are greatly reduced in proliferation and differentiation potency. Cardiovasc. Res. 83, 707–716 (2009).
Salem, B. et al. Quantitative activation suppression assay to evaluate human bone marrow-derived mesenchymal stromal cell potency. Cytotherapy 17, 1675–1686 (2015).
Cheng, K. et al. Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair. JACC Heart Fail. 2, 49–61 (2014).
Harvey, E. et al. Potency of human cardiosphere-derived cells from patients with ischemic heart disease is associated with robust vascular supportive ability. Stem Cells Transl. Med. 6, 1399–1411 (2017).
Marban, E. A mechanistic roadmap for the clinical application of cardiac cell therapies. Nat. Biomed. Eng. 2, 353–361 (2018).
MacDonald, B. T., Tamai, K. & He, X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 17, 9–26 (2009).
Cheng, K. et al. Relative roles of CD90 and c-kit to the regenerative efficacy of cardiosphere-derived cells in humans and in a mouse model of myocardial infarction. J. Am. Heart Assoc. 3, e001260 (2014).
Vargas, D. A., Sun, M., Sadykov, K., Kukuruzinska, M. A. & Zaman, M. H. The integrated role of Wnt/beta-Catenin, N-glycosylation, and E-cadherin-mediated adhesion in network dynamics. PLoS Comput. Biol. 12, e1005007 (2016).
Lin, X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development 131, 6009–6021 (2004).
Barile, L. et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 103, 530–541 (2014).
Ibrahim, A. G., Cheng, K. & Marban, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2, 606–619 (2014).
May, E., May, P. & Weil, R. Analysis of the events leading to SV40-induced chromosome replication and mitosis in primary mouse kidney cell cultures. Proc. Natl Acad. Sci. USA 68, 1208–1211 (1971).
Smith, H. S., Scher, C. D. & Todaro, G. J. Induction of cell division in medium lacking serum growth factor by SV40. Virology 44, 359–370 (1971).
Chen, T. S. et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J. Transl. Med. 9, 47 (2011).
Gillespie, J. R. et al. Deletion of glycogen synthase kinase-3beta in cartilage results in up-regulation of glycogen synthase kinase-3alpha protein expression. Endocrinology 152, 1755–1766 (2011).
Cambier, L., Plate, M., Sucov, H. M. & Pashmforoush, M. Nkx2-5 regulates cardiac growth through modulation of Wnt signaling by R-spondin3. Development 141, 2959–2971 (2014).
Li, H. et al. Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am. J. Physiol. Heart Circ. Physiol. 299, H1772–H1781 (2010).
Oh, S. Y., Kim, J. Y. & Park, C. The ETS factor, ETV2: a master regulator for vascular endothelial cell development. Mol. Cells 38, 1029–1036 (2015).
Hofsteen, P., Robitaille, A. M., Chapman, D. P., Moon & Murry, C. E. Quantitative proteomics identify DAB2 as a cardiac developmental regulator that inhibits WNT/beta-catenin signaling. Proc. Natl Acad. Sci. USA 113, 1002–1007 (2016).
Palpant, N. J. et al. Inhibition of beta-catenin signaling respecifies anterior-like endothelium into beating human cardiomyocytes. Development 142, 3198–3209 (2015).
Wang, Y. et al. Myocardial beta-catenin-BMP2 signaling promotes mesenchymal cell proliferation during endocardial cushion formation. J. Mol. Cell. Cardiol. 123, 150–158 (2018).
Klaus, A. et al. Wnt/beta-catenin and Bmp signals control distinct sets of transcription factors in cardiac progenitor cells. Proc. Natl Acad. Sci. USA 109, 10921–10926 (2012).
Zelarayan, L., Gehrke, C. & Bergmann, M. W. Role of beta-catenin in adult cardiac remodeling. Cell Cycle 6, 2120–2126 (2007).
Ghosh-Choudhury, N., Abboud, S. L., Chandrasekar, B. & Ghosh Choudhury, G. BMP-2 regulates cardiomyocyte contractility in a phosphatidylinositol 3 kinase-dependent manner. FEBS Lett. 544, 181–184 (2003).
Wang, Y. X. et al. Bone morphogenetic protein-2 acts upstream of myocyte-specific enhancer factor 2a to control embryonic cardiac contractility. Cardiovasc. Res. 74, 290–303 (2007).
Sun, Q. et al. Role of miR-17 family in the negative feedback loop of bone morphogenetic protein signaling in neuron. PLoS ONE 8, e83067 (2013).
Ning, G., Liu, X., Dai, M., Meng, A. & Wang, Q. MicroRNA-92a upholds Bmp signaling by targeting noggin3 during pharyngeal cartilage formation. Dev. Cell. 24, 283–295 (2013).
Nusse, R. Wnt signaling in disease and in development. Cell Res. 15, 28–32 (2005).
Bukowska, J., Ziecik, A. J., Laguna, J., Gawronska-Kozak, B. & Bodek, G. The importance of the canonical wnt signaling pathway in the porcine endometrial stromal stem/progenitor cells: implications for regeneration. Stem Cells Dev. 24, 2873–2885 (2015).
Cosin-Roger, J. et al. The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. Mucosal Immunol. 9, 986–998 (2016).
Li, M. et al. miR-709 modulates LPS-induced inflammatory response through targeting GSK-3beta. Int. Immunopharmacol. 36, 333–338 (2016).
Ortiz-Masia, D. et al. Hypoxic macrophages impair autophagy in epithelial cells through Wnt1: relevance in IBD. Mucosal Immunol. 7, 929–938 (2014).
Hsu, Y. C. et al. Protective effects of miR-29a on diabetic glomerular dysfunction by modulation of DKK1/Wnt/beta-catenin signaling. Sci. Rep. 6, 30575 (2016).
Zhang, G. Y. et al. A novel regulatory function for miR-29a in keloid fibrogenesis. Clin. Exp. Dermatol. 41, 341 (2016).
Chilosi, M. et al. Epithelial to mesenchymal transition-related proteins ZEB1, beta-catenin, and beta-tubulin-III in idiopathic pulmonary fibrosis. Mod. Pathol. 30, 26–38 (2016).
Wang, S. et al. GSK-3beta inhibitor CHIR-99021 promotes proliferation via upregulating beta-catenin in neonatal atrial human cardiomyocytes. J. Cardiovasc. Pharmacol. 68, 425–432 (2016).
Ni, W. et al. Extensive supporting cell proliferation and mitotic hair cell generation by in vivo genetic reprogramming in the neonatal mouse Cochlea. J. Neurosci. 36, 8734 (2016).
D’Uva, G. et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 17, 627–638 (2015).
Potz, B. A. et al. Glycogen synthase kinase 3beta inhibition improves myocardial angiogenesis and perfusion in a swine model of metabolic syndrome. J. Am. Heart Assoc. 5, e003694 (2016).
Birdsey, G. M. et al. The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/beta-catenin signaling. Dev. Cell. 32, 82–96 (2015).
Kim, K. I. et al. Beta-catenin overexpression augments angiogenesis and skeletal muscle regeneration through dual mechanism of vascular endothelial growth factor-mediated endothelial cell proliferation and progenitor cell mobilization. Arterioscler. Thromb. Vasc. Biol. 26, 91–98 (2006).
Li, J. et al. Remote preconditioning provides potent cardioprotection via PI3K/Akt activation and is associated with nuclear accumulation of beta-catenin. Clin. Sci. 120, 451 (2011).
Hahn, J. Y. et al. Beta-catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts. J. Bio. l Chem. 281, 30979–30989 (2006).
Marban, E. The secret life of exosomes: what bees can teach us about next-generation therapeutics. J. Am. Coll. Cardiol. 71, 193–200 (2018).
Dodson, B. P. & Levine, A. D. Challenges in the translation and commercialization of cell therapies. BMC Biotechnol. 15, 70 (2015).
Akers, J. C. et al. Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer Biomark. 17, 125–132 (2016).
Ibrahim, A. & Marban, E. Exosomes: fundamental biology and roles in cardiovascular physiology. Annu. Rev. Physiol. 78, 67–83 (2016).
Liu, B. et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2, 293–303 (2018).
Cambier, L. et al. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol. Med. 9, 337–352 (2017).
Gallet, R. et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 38, 201–211 (2017).
Acknowledgements
We thank the Cedars-Sinai Genomics Core for RNA sequencing, the Cedars Sinai Biobank and Translational Research Core for tissue processing, R. Benhaghnazar for technical assistance and A. Burtnick for help with Fig. 7. Work in the Marbán lab was supported by NIH R01124074; work at Capricor Therapeutics was supported by DOD PRMRP PR150618.
Author information
Authors and Affiliations
Contributions
A.I. conceived the idea, designed the experiments, performed the experiments, analysed the data and wrote the manuscript. C.L., R.R., M.F., T.A. and R.R.S. performed the experiments and provided technical and design input. L.L., S.D.V., J.J.M., B.T., A.A. and L.S. performed the experiments and analysed the data. L.R.-B. assisted with project design, L.M. supervised the study and E.M. conceived the idea, wrote the manuscript and supervised the study.
Corresponding authors
Ethics declarations
Competing interests
E.M. owns founder’s stock in Capricor Therapeutics. L.L., S.D.V., J.J.M., L.R.-B., R.R.S. and L.M. are employees of the company.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables
Rights and permissions
About this article
Cite this article
Ibrahim, A.G.E., Li, C., Rogers, R. et al. Augmenting canonical Wnt signalling in therapeutically inert cells converts them into therapeutically potent exosome factories. Nat Biomed Eng 3, 695–705 (2019). https://doi.org/10.1038/s41551-019-0448-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0448-6
This article is cited by
-
Single-cell transcriptomics reveal extracellular vesicles secretion with a cardiomyocyte proteostasis signature during pathological remodeling
Communications Biology (2023)
-
Can Extracellular Vesicles as Drug Delivery Systems Be a Game Changer in Cardiac Disease?
Pharmaceutical Research (2023)
-
Extracellular Vesicles as a Cell-free Therapy for Cardiac Repair: a Systematic Review and Meta-analysis of Randomized Controlled Preclinical Trials in Animal Myocardial Infarction Models
Stem Cell Reviews and Reports (2022)
-
Regulating the production and biological function of small extracellular vesicles: current strategies, applications and prospects
Journal of Nanobiotechnology (2021)
-
Single-cell transcriptomics of cardiac progenitors reveals functional subpopulations and their cooperative crosstalk in cardiac repair
Protein & Cell (2021)