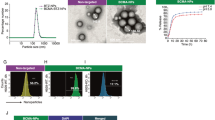
Abstract
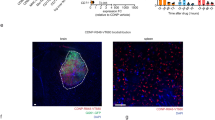
Approximately 15–40% of all cancers develop metastases in the central nervous system (CNS), yet few therapeutic options exist to treat them. Cancer therapies based on monoclonal antibodies are widely successful, yet have limited efficacy against CNS metastases, owing to the low levels of the drug reaching the tumour site. Here, we show that the encapsulation of rituximab within a crosslinked zwitterionic polymer layer leads to the sustained release of rituximab as the crosslinkers are gradually hydrolysed, enhancing the CNS levels of the antibody by approximately tenfold with respect to the administration of naked rituximab. When the nanocapsules were functionalized with CXCL13—the ligand for the chemokine receptor CXCR5, which is frequently found on B-cell lymphoma—a single dose led to improved control of CXCR5-expressing metastases in a murine xenograft model of non-Hodgkin lymphoma, and eliminated lymphoma in a xenografted humanized bone marrow–liver–thymus mouse model. Encapsulation and molecular targeting of therapeutic antibodies could become an option for the treatment of cancers with CNS metastases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all data supporting the results of this study are available within the paper and its Supplementary Information files. Source data collected in this study are available from the corresponding author upon request.
References
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 (2002).
Aragon-Ching, J. B. & Zujewski, J. A. CNS metastasis: an old problem in a new guise. Clin. Cancer Res. 13, 1644–1647 (2007).
Tosoni, A., Ermani, M. & Brandes, A. A. The pathogenesis and treatment of brain metastases: a comprehensive review. Crit. Rev. Oncol. Hematol. 52, 199–215 (2004).
Rubenstein, J. L. et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood 101, 466–468 (2003).
Zhang, Y. & Pardridge, W. M. Mediated efflux of IgG molecules from brain to blood across the blood–brain barrier. J. Neuroimmunol. 114, 168–172 (2001).
Czyzewski, K. et al. Intrathecal therapy with rituximab in central nervous system involvement of post-transplant lymphoproliferative disorder. Leuk. Lymphoma 54, 503–506 (2013).
Lu, N. T. et al. Intrathecal trastuzumab: immunotherapy improves the prognosis of leptomeningeal metastases in HER-2+ breast cancer patient. J. Immunother. Cancer 3, 41 (2015).
Cooper, P. R. et al. Efflux of monoclonal antibodies from rat brain by neonatal Fc receptor, FcRn. Brain Res. 1534, 13–21 (2013).
Rubenstein, J. L. et al. Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood 121, 745–751 (2013).
Rubenstein, J. L. et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J. Clin. Oncol. 25, 1350–1356 (2007).
Bousquet, G. et al. Intrathecal trastuzumab halts progression of CNS metastases in breast cancer. J. Clin. Oncol. 34, e151–e155 (2016).
Chen, Y. & Liu, L. Modern methods for delivery of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 64, 640–665 (2012).
Schroeder, A. et al. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer 12, 39–50 (2011).
Lu, C. T. et al. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomed. 9, 2241–2257 (2014).
Fu, H. & McCarty, D. M. Crossing the blood–brain-barrier with viral vectors. Curr. Opin. Virol. 21, 87–92 (2016).
Spencer, B. J. & Verma, I. M. Targeted delivery of proteins across the blood–brain barrier. Proc. Natl Acad. Sci. USA 104, 7594–7599 (2007).
Battaglia, L. & Gallarate, M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 9, 497–508 (2012).
Helm, F. & Fricker, G. Liposomal conjugates for drug delivery to the central nervous system. Pharmaceutics 7, 27–42 (2015).
Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv. Drug Deliv. Rev. 71, 2–14 (2014).
Mishra, B., Patel, B. B. & Tiwari, S. Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine 6, 9–24 (2010).
Papasani, M. R., Wang, G. & Hill, R. A. Gold nanoparticles: the importance of physiological principles to devise strategies for targeted drug delivery. Nanomedicine 8, 804–814 (2012).
Clark, A. J. & Davis, M. E. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc. Natl Acad. Sci. USA 112, 12486–12491 (2015).
Bharali, D. J. et al. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc. Natl Acad. Sci. USA 102, 11539–11544 (2005).
Pardridge, W. M. Drug and gene targeting to the brain with molecular Trojan horses. Nat. Rev. Drug Discov. 1, 131–139 (2002).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Rezvani, A. R. & Maloney, D. G. Rituximab resistance. Best. Pract. Res. Clin. Haematol. 24, 203–216 (2011).
Abes, R., Gelize, E., Fridman, W. H. & Teillaud, J. L. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood 116, 926–934 (2010).
Yan, M. et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat. Nanotechnol. 5, 48–53 (2010).
Tian, H. et al. Growth-factor nanocapsules that enable tunable controlled release for bone regeneration. ACS Nano 10, 7362–7369 (2016).
Liang, S. et al. Phosphorylcholine polymer nanocapsules prolong the circulation time and reduce the immunogenicity of therapeutic proteins. Nano Res. 9, 1022–1031 (2016).
Zhang, L. et al. Prolonging the plasma circulation of proteins by nanoencapsulation with phosphorylcholine-based polymer. Nano Res. 9, 2424–2432 (2016).
Young, G., Bowers, R., Hall, B. & Port, M. Six month clinical evaluation of a biomimetic hydrogel contact lens. CLAO J. 23, 226–236 (1997).
Chen, S., Li, L., Zhao, C. & Zheng, J. Surface hydration: principles and applications toward low-fouling/nonfouling biomaterials. Polymer 51, 5283–5293 (2010).
Kato, Y. et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 13, 89 (2013).
Corbet, C. & Feron, O. Tumour acidosis: from the passenger to the driver’s seat. Nat. Rev. Cancer 17, 577–593 (2017).
Ito, D. et al. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 57, 1–9 (1998).
Rosenberg, G. A. et al. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 893, 104–112 (2001).
Widney, D. et al. Levels of murine, but not human, CXCL13 are greatly elevated in NOD-SCID mice bearing the AIDS-associated Burkitt lymphoma cell line, 2F7. PLoS ONE 8, e72414 (2013).
Legler, D. F. et al. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 187, 655–660 (1998).
Gunn, M. D. et al. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature 391, 799–803 (1998).
Charbonneau, B. et al. CXCR5 polymorphisms in non-Hodgkin lymphoma risk and prognosis. Cancer Immunol. Immunother. 62, 1475–1484 (2013).
Hussain, S. K. et al. Serum levels of the chemokine CXCL13, genetic variation in CXCL13 and its receptor CXCR5, and HIV-associated non-Hodgkin B-cell lymphoma risk. Cancer Epidemiol. Biomarkers Prev. 22, 295–307 (2013).
Dobner, T., Wolf, I., Emrich, T. & Lipp, M. Differentiation-specific expression of a novel G protein-coupled receptor from Burkitt’s lymphoma. Eur. J. Immunol. 22, 2795–2799 (1992).
Smith, J. R. et al. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood 101, 815–821 (2003).
Trentin, L. et al. Homeostatic chemokines drive migration of malignant B cells in patients with non-Hodgkin lymphomas. Blood 104, 502–508 (2004).
Shultz, L. et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174, 6477–6489 (2005).
Chijioke, O. et al. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein–Barr virus infection. Cell Rep. 5, 1489–1498 (2013).
Melkus, M. et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 12, 1316–1322 (2006).
Shultz, L. D., Brehm, M. A., Garcia-Martinez, J. V. & Greiner, D. L. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 12, 786–798 (2012).
Walsh, N. C. et al. Humanized mouse models of clinical disease. Annu. Rev. Pathol. 12, 187–215 (2017).
Honeycutt, J. B. et al. Macrophages sustain HIV replication in vivo independently of T cells. J. Clin. Invest. 126, 1353–1366 (2016).
Wu, D. et al. A bioinspired platform for effective delivery of protein therapeutics to the central nervous system. Adv. Mater. 31, 1807557 (2019).
Gasque, P. et al. Complement components of the innate immune system in health and disease in the CNS. Immunopharmacology 49, 171–186 (2000).
Salter, M. W. & Beggs, S. Sublime microglia: expanding roles for the guardians of the CNS. Cell 158, 15–24 (2014).
Prinz, M. & Priller, J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 15, 300–312 (2014).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007).
Lin, N. U. et al. CNS metastases in breast cancer: old challenge, new frontiers. Clin. Cancer Res. 19, 6404–6418 (2013).
Zagouri, F. et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res. Treat. 139, 13–22 (2013).
Strayer, D. S. & Kohler, H. Immune response to phosphorylcholine II, natural “auto”-anti-receptor antibody in neonatal BALB/c mice. Cell Immunol. 25, 294–301 (1976).
Su, J. et al. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis 188, 160–166 (2006).
Bertolini, F. et al. Endostatin, an antiangiogenic drug, induces tumor stabilization after chemotherapy or anti-CD20 therapy in a NOD/SCID mouse model of human high-grade non-Hodgkin lymphoma. Blood 96, 282–287 (2000).
Zhen, A. et al. Stem-cell based engineered immunity against HIV infection in the humanized mouse model. J. Vis. Exp. 113, e54048 (2016).
Zhen, A. et al. HIV-specific immunity derived from chimeric antigen receptor-engineered stem cells. Mol. Ther. 23, 1358–1367 (2015).
Ringpis, G. et al. Engineering HIV-1-resistant T-cells from short-hairpin RNA-expressing hematopoietic stem/progenitor cells in humanized BLT mice. PLoS ONE 7, e53492 (2012).
Acknowledgements
This work was supported by the UCLA AIDS Institute HIV Extinction Project funded by the McCarthy Family Foundation (to M.K.), NIH grants RO1 CA232015 and RO1 AI110200 (to M.K.), U19AI117941 (to I.S.Y.C.) and R21 AI114433 (to I.S.Y.C.), and the California HIV/AIDS Research Grants Program (ID15-LA-050 to D.Widney). Equipment located at the UCLA AIDS Institute is supported by the James B. Pendleton Charitable Trust. Cell sorting was performed in the CFAR Flow Cytometry Core Facility supported by NIH grants P30 CA016042 and 5P30 AI028697.
Author information
Authors and Affiliations
Contributions
J.W. and M.K. contributed to the study design, performed the experiments, analysed the data and wrote the paper. D.Wu, M.Q., C.L., L.W., D.X., H.V.V., Y.Liu, E.K., X.G, G.S., Y.Lee and X.S. performed the experiments. D.Widney, O.M.-M. and Y.Lu. interpreted the data. I.S.Y.C. wrote the paper and interpreted the data. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
I.S.Y.C. has a financial interest in CSL Behring. The remaining authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures and references.
Rights and permissions
About this article
Cite this article
Wen, J., Wu, D., Qin, M. et al. Sustained delivery and molecular targeting of a therapeutic monoclonal antibody to metastases in the central nervous system of mice. Nat Biomed Eng 3, 706–716 (2019). https://doi.org/10.1038/s41551-019-0434-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0434-z
This article is cited by
-
Drug delivery methods for cancer immunotherapy
Drug Delivery and Translational Research (2024)
-
Preparation and characterization of enzyme-responsive zwitterionic nanoparticles for monoclonal antibody delivery
Frontiers of Materials Science (2023)
-
Protein nanocapsules based vectors for efficient gene transfection
Nano Research (2022)
-
Longitudinal clonal tracking in humanized mice reveals sustained polyclonal repopulation of gene-modified human-HSPC despite vector integration bias
Stem Cell Research & Therapy (2021)
-
Drug delivery to the central nervous system
Nature Reviews Materials (2021)