Abstract
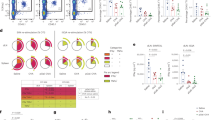
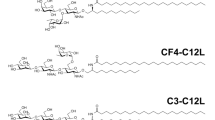
Homeostatic antigen presentation by hepatic antigen-presenting cells, which results in tolerogenic T-cell education, could be exploited to induce antigen-specific immunological tolerance. Here we show that antigens modified with polymeric forms of either N-acetylgalactosamine or N-acetylglucosamine target hepatic antigen-presenting cells, increase their antigen presentation and induce antigen-specific tolerance, as indicated by CD4+ and CD8+ T-cell deletion and anergy. These synthetically glycosylated antigens also expanded functional regulatory T cells, which are necessary for the durable suppression of antigen-specific immune responses. In an adoptive-transfer mouse model of type-1 diabetes, treatment with the glycosylated autoantigens prevented T-cell-mediated diabetes, expanded antigen-specific regulatory T cells and resulted in lasting tolerance to a subsequent challenge with activated diabetogenic T cells. Glycosylated autoantigens targeted to hepatic antigen-presenting cells might enable therapies that promote immune tolerance in patients with autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all data supporting the results in this study are available within the paper and its Supplementary Information. The datasets generated and analysed during the study are available from the corresponding author upon reasonable request.
References
Rosenblum, M. D., Gratz, I. K., Paw, J. S. & Abbas, A. K. Treating human autoimmunity: current practice and future prospects. Sci. Transl. Med. 4, 125sr1–125sr1 (2012).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Wing, K. & Sakaguchi, S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 11, 7–13 (2010).
Wang, X., Lu, L. & Jiang, S. Regulatory T cells: customizing for the clinic. Sci. Transl. Med. 3, 83ps19–83ps19 (2011).
Berg, M. et al. Cross-presentation of antigens from apoptotic tumor cells by liver sinusoidal endothelial cells leads to tumor-specific CD8+ T cell tolerance. Eur. J. Immunol. 36, 2960–2970 (2006).
Li, F. & Tian, Z. The liver works as a school to educate regulatory immune cells. Cell. Mol. Immunol. 10, 292–302 (2013).
Horst, A. K., Neumann, K., Diehl, L. & Tiegs, G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell. Mol. Immunol. 13, 277–292 (2016).
Knolle et al. IL‐10 down‐regulates T cell activation by antigen‐presenting liver sinusoidal endothelial cells through decreased antigen uptake via the mannose receptor and lowered surface expression of accessory molecules. Clin. Exp. Immunol. 114, 427–433 (1998).
Knolle, P. A. et al. Endotoxin down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells. J. Immunol. 162, 1401–1407 (1999).
Bissell, D. M., Wang, S. S., Jarnagin, W. R. & Roll, F. J. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J. Clin. Invest. 96, 447–455 (1995).
Breous, E., Somanathan, S., Vandenberghe, L. H. & Wilson, J. M. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology 50, 612–621 (2009).
Schon, H.-T. & Weiskirchen, R. Immunomodulatory effects of transforming growth factor-β in the liver. Hepatobiliary Surg. Nutr. 3, 386–406 (2014).
Chen, W. et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Wu, K., Kryczek, I., Chen, L., Zou, W. & Welling, T. H. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res 69, 8067–8075 (2009).
Dolina, J. S., Sung, S.-S. J., Novobrantseva, T. I., Nguyen, T. M. & Hahn, Y. S. Lipidoid nanoparticles containing PD-L1 siRNA delivered in vivo enter Kupffer cells and enhance NK and CD8+ T cell-mediated hepatic antiviral immunity. Mol. Ther. Nucleic Acids 2, e72 (2013).
Xia, C.-Q., Campbell, K., Keselowsky, B. & Clare-Salzler, M. in Type 1 Diabetes—Pathogenesis, Genetics and Immunotherapy (ed. Wagner, D.) https://doi.org/10.5772/22113 (InTech, 2011).
Bilyy, R. & Stoika, R. Search for novel cell surface markers of apoptotic cells. Autoimmunity 40, 249–253 (2009).
Duvall, E., Wyllie, A. H. & Morris, R. G. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology 56, 351–358 (1985).
Liu, W. et al. Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J. Biol. Chem. 279, 18748–18758 (2004).
Apostolopoulos, V., Thalhammer, T., Tzakos, A. G. & Stojanovska, L. Targeting antigens to dendritic cell receptors for vaccine development. J. Drug Deliv. 2013, 869718 (2013).
van Kooyk, Y. C-type lectins on dendritic cells: key modulators for the induction of immune responses. Biochem. Soc. Trans. 36, 1478–1481 (2008).
Ohnishi, H., Oka, K., Mizuno, S. & Nakamura, T. Identification of mannose receptor as receptor for hepatocyte growth factor β-chain: novel ligand-receptor pathway for enhancing macrophage phagocytosis. J. Biol. Chem. 287, 13371–13381 (2012).
Yang, C.-Y. et al. CLEC4F is an inducible C-type lectin in F4/80-positive cells and is involved in alpha-galactosylceramide presentation in liver. PLoS ONE 8, e65070 (2013).
Elvevold, K. et al. Liver sinusoidal endothelial cells depend on mannose receptor-mediated recruitment of lysosomal enzymes for normal degradation capacity. Hepatology 48, 2007–2015 (2008).
Greco, S. H. et al. Mincle signaling promotes Con A hepatitis. J. Immunol. 197, 2816–2827 (2016).
Maynard, Y. & Baenziger, J. U. Characterization of a mannose and N-acetylglucosamine-specific lectin present in rat hepatocytes. J. Biol. Chem. 257, 3788–3794 (1982).
Kim, S. H., Goto, M. & Akaike, T. Specific binding of glucose-derivatized polymers to the asialoglycoprotein receptor of mouse primary hepatocytes. J. Biol. Chem. 276, 35312–35319 (2001).
Jackson, D. C., Drummer, H. E., Urge, L., Otvos, L. Jr & Brown, L. E. Glycosylation of a synthetic peptide representing a T-cell determinant of influenza virus hemagglutinin results in loss of recognition by CD4+ T-cell clones. Virology 199, 422–430 (1994).
Hastings, K. T. & Cresswell, P. Disulfide reduction in the endocytic pathway: immunological functions of gamma-interferon-inducible lysosomal thiol reductase. Antioxid. Redox Signal. 15, 657–668 (2011).
Arunachalam, B., Phan, U. T., Geuze, H. J. & Cresswell, P. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT). Proc. Natl Acad. Sci. USA 97, 745–750 (2000).
Satyam, A. Design and synthesis of releasable folate-drug conjugates using a novel heterobifunctional disulfide-containing linker. Bioorg. Med. Chem. Lett. 18, 3196–3199 (2008).
Harvey, D. J., Wing, D. R., Küster, B. & Wilson, I. B. H. Composition of N-linked carbohydrates from ovalbumin and co-purified glycoproteins. J. Am. Soc. Mass Spectrom. 11, 564–571 (2000).
Kindberg, G. M., Magnusson, S., Berg, T. & Smedsrød, B. Receptor-mediated endocytosis of ovalbumin by two carbohydrate-specific receptors in rat liver cells. The intracellular transport of ovalbumin to lysosomes is faster in liver endothelial cells than in parenchymal cells. Biochem. J. 270, 197–203 (1990).
Schurich, A. et al. Distinct kinetics and dynamics of cross-presentation in liver sinusoidal endothelial cells compared to dendritic cells. Hepatology 50, 909–919 (2009).
Rensen, P. C. et al. Determination of the upper size limit for uptake and processing of ligands by the asialoglycoprotein receptor on hepatocytes in vitro and in vivo. J. Biol. Chem. 276, 37577–37584 (2001).
Eickmeier, I. et al. Influence of CD8 T cell priming in liver and gut on the enterohepatic circulation. J. Hepatol. 60, 1143–1150 (2014).
Okazaki, T. & Honjo, T. The PD-1–PD-L pathway in immunological tolerance. Trends Immunol. 27, 195–201 (2006).
Tokita, D. et al. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation 85, 369–377 (2008).
Crespo, J., Sun, H., Welling, T. H., Tian, Z. & Zou, W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 25, 214–221 (2013).
Carambia, A. et al. TGF-β-dependent induction of CD4+CD25+Foxp3+ Tregs by liver sinusoidal endothelial cells. J. Hepatol. 61, 594–599 (2014).
Heymann, F. et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 62, 279–291 (2015).
Grinberg-Bleyer, Y. et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J. Exp. Med. 207, 20100209 (2010).
Maloy, K. J. & Powrie, F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat. Immunol. 6, 1071–1072 (2005).
Schurich, A. et al. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J. Immunol. 184, 4107–4114 (2010).
Tye, G. J. et al. The combined molecular adjuvant CASAC enhances the CD8+ T cell response to a tumor-associated self-antigen in aged, immunosenescent mice. Immun. Ageing 12, 659 (2015).
Zelenay, S. & Demengeot, J. Comment on ‘cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells’. J. Immunol. 177, 2036–2037 (2006).
Kohm, A. P. et al. Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol. 176, 3301–3305 (2006).
Baynes, J. W. & Wold, F. Effect of glycosylation on the in vivo circulating half-life of ribonuclease. J. Biol. Chem. 251, 6016–6024 (1976).
Petzold, C. et al. Foxp3+ regulatory T cells in mouse models of type 1 diabetes. J. Diabetes Res. 2013, 940710–940710 (2013).
Delong, T. et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 351, 711–714 (2016).
Carambia, A. et al. Nanoparticle-based autoantigen delivery to Treg-inducing liver sinusoidal endothelial cells enables control of autoimmunity in mice. J. Hepatol. 62, 1349–1356 (2015).
Estey, T., Kang, J., Schwendeman, S. P. & Carpenter, J. F. BSA degradation under acidic conditions: a model for protein instability during release from PLGA delivery systems. J. Pharm. Sci. 95, 1626–1639 (2006).
Prior, S. et al. In vitro phagocytosis and monocyte-macrophage activation with poly(lactide) and poly(lactide-co-glycolide) microspheres. Eur. J. Pharm. Sci. 15, 197–207 (2002).
Wisse, E., Jacobs, F., Topal, B., Frederik, P. & De Geest, B. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 15, 1193–1199 (2008).
Perdicchio, M. et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc. Natl Acad. Sci. USA 113, 3329–3334 (2016).
Chen, P. et al. Dendritic cell targeted vaccines: recent progresses and challenges. Hum. Vaccin. Immunother. 12, 612–622 (2015).
Harding, F. A., Stickler, M. M., Razo, J. & DuBridge, R. B. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs 2, 256–265 (2010).
Knolle, P. A. & Wohlleber, D. Immunological functions of liver sinusoidal endothelial cells. Cell. Mol. Immunol. 13, 347–353 (2016).
Tang, L. et al. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology 137, 1498–1508.e5 (2009).
Domínguez-Soto, A. et al. The pathogen receptor liver and lymph node sinusoidal endotelial cell C-type lectin is expressed in human Kupffer cells and regulated by PU.1. Hepatology 49, 287–296 (2009).
Daniels, C. K., Schmucker, D. L. & Jones, A. L. Hepatic asialoglycoprotein receptor-mediated binding of human polymeric immunoglobulin A. Hepatology 9, 229–234 (1989).
Acknowledgements
We thank the Flow Cytometry Core Facility of EPFL for technical assistance and E. Simeoni (EPFL) for helpful discussion on the research and guidance on animal work. D.S.W. was supported by a fellowship from the Whitaker Foundation. This study was supported by the School of Life Sciences, EPFL, the University of Chicago, the Juvenile Diabetes Research Foundation and Anokion.
Author information
Authors and Affiliations
Contributions
D.S.W. and J.A.H. designed the research; D.S.W. and M.M.R. performed synthesis; D.S.W., M.D., S.H., K.B., G.D. and X.Q.-T. performed biological research; D.S.W. analysed data and D.S.W. and J.A.H. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The EPFL has filed for patent protection on the p(GalNAc) and p(GluNAc) delivery platforms and D.S.W. and J.A.H. are named as inventors on the patents. Anokion and Kanyos Bio have licensed the patents and J.A.H. and D.S.W. participate in equity in these companies.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods and figures.
Rights and permissions
About this article
Cite this article
Wilson, D.S., Damo, M., Hirosue, S. et al. Synthetically glycosylated antigens induce antigen-specific tolerance and prevent the onset of diabetes. Nat Biomed Eng 3, 817–829 (2019). https://doi.org/10.1038/s41551-019-0424-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0424-1
This article is cited by
-
Tolerance-inducing therapies in coeliac disease — mechanisms, progress and future directions
Nature Reviews Gastroenterology & Hepatology (2024)
-
The therapeutic potential of immunoengineering for systemic autoimmunity
Nature Reviews Rheumatology (2024)
-
Engineering the lymph node environment promotes antigen-specific efficacy in type 1 diabetes and islet transplantation
Nature Communications (2023)
-
Synthetically glycosylated antigens for the antigen-specific suppression of established immune responses
Nature Biomedical Engineering (2023)
-
Induction of antigen-specific tolerance by nanobody–antigen adducts that target class-II major histocompatibility complexes
Nature Biomedical Engineering (2021)