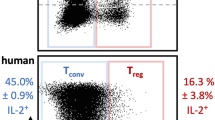
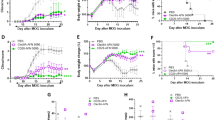
Abstract
Targeted suppression of autoimmune diseases without collateral suppression of normal immunity remains an elusive yet clinically important goal. Targeted blockade of programmed-cell-death-protein-1 (PD-1)—an immune checkpoint factor expressed by activated T cells and B cells—is an efficacious therapy for potentiating immune activation against tumours. Here we show that an immunotoxin consisting of an anti-PD-1 single-chain variable fragment, an albumin-binding domain and Pseudomonas exotoxin targeting PD-1-expressing cells, selectively recognizes and induces the killing of the cells. Administration of the immunotoxin to mouse models of autoimmune diabetes delays disease onset, and its administration in mice paralysed by experimental autoimmune encephalomyelitis ameliorates symptoms. In all mouse models, the immunotoxin reduced the numbers of PD-1-expressing cells, of total T cells and of cells of an autoreactive T-cell clone found in inflamed organs, while maintaining active adaptive immunity, as evidenced by full-strength immune responses to vaccinations. The targeted depletion of PD-1-expressing cells contingent to the preservation of adaptive immunity might be effective in the treatment of a wide range of autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all other data supporting the findings of this study are available within the paper and its Supplementary Information. Source data for the figures and encoding genes are available at figshare (https://figshare.com/s/f14f13bf582ce99165a1)63.
References
Bluestone, J. A., Herold, K. & Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464, 1293–1300 (2010).
Atkinson, M. A., Eisenbarth, G. S. & Michels, A. W. Type 1 diabetes. Lancet 383, 69–82 (2014).
Laurence, A. & Aringer, M. in The Autoimmune Diseases 5th edn (eds Mackay, I. & Rose, N. R.) 311–318 (Elsevier, San Diego, 2014).
van Belle, T. L., Coppieters, K. T. & von Herrath, M. G. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol. Rev. 91, 79–118 (2011).
Farber, R., Harel, A. & Lublin, F. Novel agents for relapsing forms of multiple sclerosis. Ann. Rev. Med. 67, 309–321 (2016).
Wingerchuk, D. M. & Carter, J. L. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin. Proc. 89, 225–240 (2014).
Kim, S. S., Kirou, K. A. & Erkan, D. Belimumab in systemic lupus erythematosus: an update for clinicians. Ther. Adv. Chronic Dis. 3, 11–23 (2012).
Chatenoud, L. in The Autoimmune Diseases 5th edn (eds Mackay, I. & Rose, N. R.) 1121–1145 (Elesvier, San Diego, 2014).
Elsegeiny, W., Eddens, T., Chen, K. & Kolls, J. K. Anti-CD20 antibody therapy and susceptibility to Pneumocystis pneumonia. Infect. Immun. 83, 2043–2052 (2015).
Sanofi. Lemtrada (alemtuzumab): Highlights of prescribing information. (FDA, 2019); http://products.sanofi.us/lemtrada/lemtrada.html.
Torkildsen, Ø., Myhr, K. M. & Bø, L. Disease-modifying treatments for multiple sclerosis—a review of approved medications. Eur. J. Neurol. 23, 18–27 (2016).
McNamara, C., Sugrue, B. & MacMahon, P. J. Current and emerging therapies in multiple sclerosis: implications for the radiologist, part 2—surveillance for treatment complications and disease progression. Am. J. Neuroradiol. 38, 1672–1680 (2017).
Francisco, L. M., Sage, P. T. & Sharpe, A. H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236, 219–242 (2010).
Agata, Y. et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 8, 765–772 (1996).
Yamazaki, T. et al. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 169, 5538–5545 (2002).
Joller, N., Peters, A., Anderson, A. C. & Kuchroo, V. K. Immune checkpoints in central nervous system autoimmunity. Immunol. Rev. 248, 122–139 (2012).
Liang, S. C. et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 33, 2706–2716 (2003).
Zhu, B. et al. Differential role of programmed death-ligand 1 and programmed death-ligand 2 in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J. Immunol. 176, 3480–3489 (2006).
Salama, A. D. et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J. Exp. Med. 198, 71–78 (2003).
Latchman, Y. E. et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl Acad. Sci. USA 101, 10691–10696 (2004).
Okazaki, T., Chikuma, S., Iwai, Y., Fagarasan, S. & Honjo, T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 14, 1212–1218 (2013).
Keir, M. E., Butte, M. J., Freeman, G. J. & Sharpe, A. H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Fife, B. T. et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 10, 1185–1192 (2009).
Godwin, J. L. et al. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J. Immunother. Cancer 5, 40 (2017).
Ansari, M. J. et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 198, 63–69 (2003).
Hughes, J. et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care 38, e55–e57 (2015).
Zhao, P. et al. An anti-programmed death-1 antibody (αPD-1) fusion protein that self-assembles into a multivalent and functional αPD-1 nanoparticle. Mol. Pharm. 14, 1494–1500 (2017).
Levy, O. E. et al. Novel exenatide analogs with peptidic albumin binding domains: potent anti-diabetic agents with extended duration of action. PLoS ONE 9, e87704 (2014).
Wang, P. et al. An albumin binding polypeptide both targets cytotoxic T lymphocyte vaccines to lymph nodes and boosts vaccine presentation by dendritic cells. Theranostics 8, 223–236 (2018).
Weldon, J. E. et al. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol. Cancer Ther. 12, 48–57 (2013).
Onda, M. et al. Recombinant immunotoxin against B-cell malignancies with no immunogenicity in mice by removal of B-cell epitopes. Proc. Natl Acad. Sci. USA 108, 5742–5747 (2011).
Chen, H. et al. Chemical conjugation of evans blue derivative: a strategy to develop long-acting therapeutics through albumin binding. Theranostics 6, 243–253 (2016).
Hassan, R., Alewine, C. & Pastan, I. New life for immunotoxin cancer therapy. Clin. Cancer Res. 22, 1055–1058 (2016).
Jonsson, A., Dogan, J., Herne, N., Abrahmsen, L. & Nygren, P. A. Engineering of a femtomolar affinity binding protein to human serum albumin. Protein Eng. Des. Sel. 21, 515–527 (2008).
Yamazaki, T. et al. Blockade of B7-H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-γ-induced nitric oxide production. J. Immunol. 175, 1586–1592 (2005).
Brinkmann, U. in Antibody Engineering (eds Kontermann, R. & Dübel, S.) 181–189 (Springer, Berlin, 2010).
Hirano, F. et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 65, 1089–1096 (2005).
Bera, T. K., Onda, M., Kreitman, R. J. & Pastan, I. An improved recombinant Fab-immunotoxin targeting CD22 expressing malignancies. Leuk. Res. 38, 1224–1229 (2014).
Fife, B. T. et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1–PD-L1 pathway. J. Exp. Med. 203, 2737–2747 (2006).
Brode, S., Raine, T., Zaccone, P. & Cooke, A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J. Immunol. 177, 6603–6612 (2006).
Wang, J. et al. Establishment of NOD-Pdcd1 −/− mice as an efficient animal model of type I diabetes. Proc. Natl Acad. Sci. USA 102, 11823–11828 (2005).
Wang, Y. et al. Neuropilin-1 modulates interferon-γ-stimulated signaling in brain microvascular endothelial cells. J. Cell Sci. 129, 3911–3921 (2016).
Rangachari, M. & Kuchroo, V. K. Using EAE to better understand principles of immune function and autoimmune pathology. J. Autoimmun. 45, 31–39 (2013).
Schreiner, B., Bailey, S. L., Shin, T., Chen, L. & Miller, S. D. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur. J. Immunol. 38, 2706–2717 (2008).
Massilamany, C., Upadhyaya, B., Gangaplara, A., Kuszynski, C. & Reddy, J. Detection of autoreactive CD4 T cells using major histocompatibility complex class II dextramers. BMC. Immunol. 12, 40 (2011).
Turner, M. J. et al. Immune status following alemtuzumab treatment in human CD52 transgenic mice. J. Neuroimmunol. 261, 29–36 (2013).
Sharon, R., McMaster, P. R., Kask, A. M., Owens, J. D. & Paul, W. E. DNP-Lys-Ficoll: a T-independent antigen which elicits both IgM and IgG anti-DNP antibody-secreting cells. J. Immunol. 114, 1585–1589 (1975).
Zhang, Q. & Vignali, D. A. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity 44, 1034–1051 (2016).
Sharpe, A. H. & Pauken, K. E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 18, 153–167 (2018).
Davidson, A. & Diamond, B. in The Autoimmune Diseases 5th edn (eds Mackay, I. & Rose, N. R.) 19–37 (Elsevier, San Diego, 2014).
Kasagi, S. et al. In vivo-generated antigen-specific regulatory T cells treat autoimmunity without compromising antibacterial immune response. Sci. Transl. Med. 6, 241ra278 (2014).
Dong, S., Xu, T., Wang, P., Zhao, P. & Chen, M. Engineering of a self-adjuvanted iTEP-delivered CTL vaccine. Acta Pharmacol. Sin. 38, 914–923 (2017).
Dong, S., Xu, T., Zhao, P., Parent, K. N. & Chen, M. A comparison study of iTEP nanoparticle-based CTL vaccine carriers revealed a surprise relationship between the stability and efficiency of the carriers. Theranostics 6, 666–678 (2016).
Zimmerman, T. et al. Simultaneous metal chelate affinity purification and endotoxin clearance of recombinant antibody fragments. J. Immunol. Methods 314, 67–73 (2006).
Hunter, S. A. & Cochran, J. R. Cell-binding assays for determining the affinity of protein–protein interactions: technologies and considerations. Methods Enzymol. 580, 21–44 (2016).
Shalem, O. et al. Genome-scale CRISPR–Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Pear, W. S., Nolan, G. P., Scott, M. L. & Baltimore, D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl Acad. Sci. USA 90, 8392–8396 (1993).
Zhang, Y., Huo, M., Zhou, J. & Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 99, 306–314 (2010).
Gregori, S., Giarratana, N., Smiroldo, S. & Adorini, L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J. Immunol. 171, 4040–4047 (2003).
Chitnis, T. et al. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J. Clin. Invest. 108, 739–747 (2001).
McMahon, E. J., Bailey, S. L., Castenada, C. V., Waldner, H. & Miller, S. D. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 11, 335–339 (2005).
Pino, P. A. & Cardona, A. E. Isolation of brain and spinal cord mononuclear cells using percoll gradients. J. Vis. Exp. 2, e2348 (2011).
Zhao, P. et al. Depletion of PD-1-positive cells ameliorates autoimmune disease. Figshare https://figshare.com/s/f14f13bf582ce99165a1 (2019).
Acknowledgements
We thank X. Wang and H. Dai for their assistance in breeding mice. The flow cytometry work was conducted in the Flow Cytometry Core Facility of University of Utah. We thank Y. Zhang and J. Wang for their review and comments on the statistical analysis of this study and S. Owen and A. Dixon for their technical assistance with antibody-related protein engineering. S.J.F. was supported by grants NS070235 and JDRF 2-SRA-2014–270-M-R. R.S.F. was supported by grants R01NS065714, R01NS082102 and R01NS091939. S.G.Z. was supported by grants R01AR059103 and R61AR073409, and the NIH Star Award. P.Z. was supported by a Graduate Research Fellowship from the University of Utah. This work was primarily supported by the University of Utah Start-up Fund, a Huntsman Cancer Institute Pilot Grant (Grant number 170301), and party by a NIH grant (R21EB024083) to M.C.
Author information
Authors and Affiliations
Contributions
P.Z. and M.C. wrote the manuscript with significant suggestions from S.G.Z. and S.J.F. R.S.F., P.Z. and M.C. designed all experiments and analysed all experimental data. S.J.F. and X.H. contributed to the design of the T1D studies; R.S.F. contributed to the design of the EAE studies; Z.Z. generated PD-1− EL4 cells; Y.C. assisted with pharmacokinetics analysis. P.Z. designed and prepared immunotoxin with assistance from S.D. P.Z. characterized immunotoxin in vitro and in vivo. P.W. contributed to the design of ABD and the related studies. H.Y. provided the αPD-1 hybridoma and guidance on PD-1 biology. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.C., P.Z. and P.W. have a pending patent application related to this work.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures
Rights and permissions
About this article
Cite this article
Zhao, P., Wang, P., Dong, S. et al. Depletion of PD-1-positive cells ameliorates autoimmune disease. Nat Biomed Eng 3, 292–305 (2019). https://doi.org/10.1038/s41551-019-0360-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0360-0
This article is cited by
-
Targeting pathogenic CD8+ tissue-resident T cells with chimeric antigen receptor therapy in murine autoimmune cholangitis
Nature Communications (2024)
-
High dimensional proteomic mapping of bone marrow immune characteristics in immune thrombocytopenia
Science China Life Sciences (2024)
-
Remodeling articular immune homeostasis with an efferocytosis-informed nanoimitator mitigates rheumatoid arthritis in mice
Nature Communications (2023)
-
DNA methylation and transcriptome signatures of the PDCD1 gene in ankylosing spondylitis
Genes & Immunity (2023)
-
An in situ dual-anchoring strategy for enhanced immobilization of PD-L1 to treat autoimmune diseases
Nature Communications (2023)