Abstract
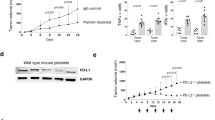
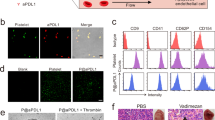
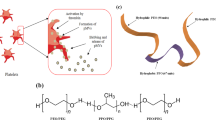
Patients with acute myeloid leukaemia who relapse following therapy have few treatment options and face poor outcomes. Immune checkpoint inhibition, for example, by antibody-mediated programmed death-1 (PD-1) blockade, is a potent therapeutic modality that improves treatment outcomes in acute myeloid leukaemia. Here, we show that systemically delivered blood platelets decorated with anti-PD-1 antibodies (aPD-1) and conjugated to haematopoietic stem cells (HSCs) suppress the growth and recurrence of leukaemia in mice. Following intravenous injection into mice bearing leukaemia cells, the HSC–platelet–aPD-1 conjugate migrated to the bone marrow and locally released aPD-1, significantly enhancing anti-leukaemia immune responses, and increasing the number of active T cells, production of cytokines and chemokines, and survival time of the mice. This cellular conjugate also promoted resistance to re-challenge with leukaemia cells. Taking advantage of the homing capability of HSCs and in situ activation of platelets for the enhanced delivery of a checkpoint inhibitor, this cellular combination-mediated drug delivery strategy can significantly augment the therapeutic efficacy of checkpoint blockade.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. Source data for the figures are available in Figshare at https://doi.org/10.6084/m9.figshare.7033481.
References
Huntly, B. J. & Gilliland, D. G. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat. Rev. Cancer 5, 311–321 (2005).
Dick, J. E. Acute myeloid leukemia stem cells. Ann. NY Acad. Sci. 1044, 1–5 (2005).
Estey, E. & Döhner, H. Acute myeloid leukaemia. Lancet 368, 1894–1907 (2006).
Döhner, H. et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European Leukemia Net. Blood 115, 453–474 (2010).
Lowenberg, B., Downing, J. R. & Burnett, A. Acute myeloid leukemia. N. Engl. J. Med. 1999, 1051–1062 (1999).
Advani, R. et al. Treatment of refractory and relapsed acute myelogenous leukemia with combination chemotherapy plus the multidrug resistance modulator PSC 833 (Valspodar). Blood 93, 787–795 (1999).
Fernandez, H. F. et al. Anthracycline dose intensification in acute myeloid leukemia. N. Engl. J. Med. 361, 1249–1259 (2009).
Leith, C. P. et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia. A Southwest Oncology Group Study. Blood 94, 1086–1099 (1999).
Gottesman, M. M., Fojo, T. & Bates, S. E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer 2, 48–58 (2002).
Leith, C. P. et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood 89, 3323–3329 (1997).
Ofran, Y. & Rowe, J. M. Treatment for relapsed acute myeloid leukemia: what is new? Curr. Opin. Hematol. 19, 89–94 (2012).
Ding, L. et al. Clonal evolution in relapsed acute myeloid leukemia revealed by whole genome sequencing. Nature 481, 506–510 (2012).
Lagasse, E. et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 6, 1229–1234 (2000).
Wilson, A. & Trumpp, A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 6, 93–106 (2006).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Ellebrecht, C. T. et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353, 179–184 (2016).
Wu, C.-Y., Roybal, K. T., Puchner, E. M., Onuffer, J. & Lim, W. A. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 350, aab4077 (2015).
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra138 (2013).
Kingwell, K. CAR T therapies drive into new terrain. Nat. Rev. Drug Discov. 16, 301–304 (2017).
Jackson, H. J., Rafiq, S. & Brentjens, R. J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 13, 370–383 (2016).
Kershaw, M. H., Westwood, J. A. & Darcy, P. K. Gene-engineered T cells for cancer therapy. Nat. Rev. Cancer 13, 525–541 (2013).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Ishida, Y., Agata, Y., Shibahara, K. & Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11, 3887–3895 (1992).
Keir, M. E. et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 203, 883–895 (2006).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Zhou, Q. et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood 116, 2484–2493 (2010).
McClanahan, F. et al. PD-L1 checkpoint blockade prevents immune dysfunction and leukemia development in a mouse model of chronic lymphocytic leukemia. Blood 126, 203–211 (2015).
Zhang, L., Gajewski, T. F. & Kline, J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 114, 1545–1552 (2009).
Kamath, S., Blann, A. & Lip, G. Platelet activation: assessment and quantification. Eur. Heart J. 22, 1561–1571 (2001).
Giralt, S. A. & Champlin, R. E. Leukemia relapse after allogeneic bone marrow transplantation: a review. Blood 84, 3603–3612 (1994).
Hu, C.-M. J. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121 (2015).
Wang, C. et al. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 1, 0011 (2017).
Hang, H. C., Yu, C., Kato, D. L. & Bertozzi, C. R. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. Proc. Natl Acad. Sci. USA 100, 14846–14851 (2003).
Shi, P. et al. Spatiotemporal control of cell–cell reversible interactions using molecular engineering. Nat. Commun. 7, 13088 (2016).
Zhao, M. et al. Clickable protein nanocapsules for targeted delivery of recombinant p53 protein. J. Am. Chem. Soc. 136, 15319–15325 (2014).
Eeftens, J. M., van der Torre, J., Burnham, D. R. & Dekker, C. Copper-free click chemistry for attachment of biomolecules in magnetic tweezers. BMC Biophys. 8, 9 (2015).
Leopold, L. H. & Willemze, R. The treatment of acute myeloid leukemia in first relapse: a comprehensive review of the literature. Leuk. Lymphoma 43, 1715–1727 (2002).
Swami, A. et al. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc. Natl Acad. Sci. USA 111, 10287–10292 (2014).
Hu, Q. et al. Engineered nanoplatelets for enhanced treatment of multiple myeloma and thrombus. Adv. Mater. 28, 9573–9580 (2016).
Ruggeri, Z. M., Orje, J. N., Habermann, R., Federici, A. B. & Reininger, A. J. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 108, 1903–1910 (2006).
Miyazaki, Y. et al. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood 88, 3456–3464 (1996).
Yan, M. & Jurasz, P. The role of platelets in the tumor microenvironment: from solid tumors to leukemia. Biochim. Biophys. Acta 1863, 392–400 (2016).
Velez, J. et al. Platelets promote mitochondrial uncoupling and resistance to apoptosis in leukemia cells: a novel paradigm for the bone marrow microenvironment. Cancer Microenviron. 7, 79–90 (2014).
Zhou, Q. et al. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood 114, 3793–3802 (2009).
Hlavacek, W. S., Posner, R. G. & Perelson, A. S. Steric effects on multivalent ligand–receptor binding: exclusion of ligand sites by bound cell surface receptors. Biophys. J. 76, 3031–3043 (1999).
Costinean, S. et al. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in Eμ-miR155 transgenic mice. Proc. Natl Acad. Sci. USA 103, 7024–7029 (2006).
Pandolfi, A. et al. PAK1 is a therapeutic target in acute myeloid leukemia and myelodysplastic syndrome. Blood 126, 1118–1127 (2015).
Moynihan, K. D. et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 22, 1402–1410 (2016).
Döhner, H., Weisdorf, D. J. & Bloomfield, C. D. Acute myeloid leukemia. N. Engl. J. Med. 373, 1136–1152 (2015).
Ayar, S. P., Ravula, S. & Polski, J. M. Granulocyte, monocyte and blast immunophenotype abnormalities in acute myeloid leukemia with myelodysplasia-related changes. Ann. Clin. Lab. Sci. 44, 3–9 (2014).
Stetler-Stevenson, M. et al. Diagnostic utility of flow cytometric immunophenotyping in myelodysplastic syndrome. Blood 98, 979–987 (2001).
Lu, Y., Aimetti, A. A., Langer, R. & Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 1, 16075 (2016).
Wang, Q. et al. Non-genetic engineering of cells for drug delivery and cell-based therapy. Adv. Drug Deliv. Rev. 91, 125–140 (2015).
Chen, Z., Hu, Q. & Gu, Z. Leveraging engineering of cells for drug delivery. Acc. Chem. Res. 51, 668–677 (2018).
Cheng, H. et al. Stem cell membrane engineering for cell rolling using peptide conjugation and tuningof cell-selectin interaction kinetics. Biomaterials 33, 5004–5012 (2012).
Acknowledgements
This work was supported by grants from the start-up packages of UNC/NC State and UCLA, the Sloan Research Fellowship of the Alfred P. Sloan Foundation, the National Key R&D Program of China (2017YFA0205600), the National Natural Science Foundation of China (51728301, 81690263) and the China Scholarship Council (CSC). We acknowledge B. Blazar at the University of Minnesota for providing the C1498-Luc cell line and M. Liu at New York University for assistance in cytokine analysis. Z.G. acknowledges support from W. Gu and P. Zhang.
Author information
Authors and Affiliations
Contributions
Q.H. and Z.G. designed the experiments. Q.H., W.S., J.W., H.R., Y.Y., X.Z. and C.W. performed the experiments and collected the data. All authors contributed to writing the manuscript, discussing the results and implications, and editing the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
Patents describing the cell-combination drug-delivery system documented in this article have been filed with the US Patent Office. Q.H. and Z.G. are inventors of the following provisional patent application: US 62/653,843. J.F.Z. has received honoraria from Agios, Celgene and Tolero, consultancy from Celgene and Asystbio Laboratories, and research funding from Merck, Takeda and Tolero. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Figures 1–35
Rights and permissions
About this article
Cite this article
Hu, Q., Sun, W., Wang, J. et al. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat Biomed Eng 2, 831–840 (2018). https://doi.org/10.1038/s41551-018-0310-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0310-2
This article is cited by
-
Immune cells: potential carriers or agents for drug delivery to the central nervous system
Military Medical Research (2024)
-
Targeted therapies of inflammatory diseases with intracellularly gelated macrophages in mice and rats
Nature Communications (2024)
-
Bionic immunoactivator copresenting autophagy promoting and costimulatory molecules for synergistic cancer immunotherapy
Nano Research (2024)
-
Development and application of nanomaterials, nanotechnology and nanomedicine for treating hematological malignancies
Journal of Hematology & Oncology (2023)
-
Sensitive and quantitative in vivo analysis of PD-L1 using magnetic particle imaging and imaging-guided immunotherapy
European Journal of Nuclear Medicine and Molecular Imaging (2023)