Abstract
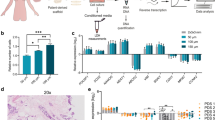
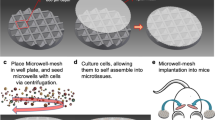
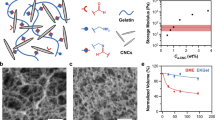
Survivors of cancer often carry disseminated tumour cells (DTCs); however, they do not relapse from treatment owing to DTC dormancy. Understanding how the local microenvironment regulates the transition of DTCs from a quiescent state to active proliferation could suggest new therapeutic strategies to prevent or delay the formation of metastases. Here, we show that implantable biomaterial microenvironments incorporating human stromal cells, immune cells and cancer cells can be used to examine the post-dissemination phase of tumour microenvironment evolution. After subdermal implantation in mice, porous hydrogel scaffolds seeded with human bone marrow stromal cells form a vascularized niche and recruit human circulating tumour cells released from an orthotopic prostate tumour xenograft. Systemic injection of human peripheral blood mononuclear cells slowed the progression of early metastatic niches. However, the rate of overt metastases did not change. Implantable pre-metastatic niches provide a new opportunity to study DTC activation and evolution to lethal metastasis, and could facilitate the development of effective anti-metastatic therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information.
References
Luzzi, K. J. et al. Multistep nature of metastatic inefficiency—dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 153, 865–873 (1998).
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 (2002).
Aguirre-Ghiso, J. A. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 7, 834–846 (2007).
Meng, S. et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 10, 8152–8162 (2004).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Sosa, M. S., Bragado, P. & Aguirre-Ghiso, J. A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611–622 (2014).
He, F. et al. Multiscale characterization of the mineral phase at skeletal sites of breast cancer metastasis. Proc. Natl Acad. Sci. USA 114, 10542–10547 (2017).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1, 571–573 (1889).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Kienast, Y. et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16, 116–122 (2010).
Wong, C. C. et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc. Natl Acad. Sci. USA 108, 16369–16374 (2011).
Oskarsson, T. et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 17, 867–874 (2011).
Barkan, D. et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 70, 5706–5716 (2010).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009).
Eyles, J. et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Invest. 120, 2030–2039 (2010).
Koebel, C. M. et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450, 903–907 (2007).
Muller-Hermelink, N. et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell 13, 507–518 (2008).
Hanna, R. N. et al. Patrolling monocytes control tumor metastasis to the lung. Science 350, 985–990 (2015).
Lambert, A. W., Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis. Cell 168, 670–691 (2017).
Kang, Y. & Pantel, K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell 23, 573–581 (2013).
Francia, G., Cruz-Munoz, W., Man, S., Xu, P. & Kerbel, R. S. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat. Rev. Cancer 11, 135–141 (2011).
Bos, P. D., Nguyen, D. X. & Massague, J. Modeling metastasis in the mouse. Curr. Opin. Pharmacol. 10, 571–577 (2010).
Shultz, L. D., Ishikawa, F. & Greiner, D. L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 (2007).
Bersani, F. et al. Bioengineered implantable scaffolds as a tool to study stromal-derived factors in metastatic cancer models. Cancer Res. 74, 7229–7238 (2014).
Villasante, A. & Vunjak-Novakovic, G. Tissue-engineered models of human tumors for cancer research. Expert Opin. Drug Discov. 10, 257–268 (2015).
Hutmacher, D. W. et al. Can tissue engineering concepts advance tumor biology research? Trends Biotechnol. 28, 125–133 (2010).
Lee, J., Cuddihy, M. J. & Kotov, N. A. Three-dimensional cell culture matrices: state of the art. Tissue Eng. Part B Rev. 14, 61–86 (2008).
Seib, F. P., Berry, J. E., Shiozawa, Y., Taichman, R. S. & Kaplan, D. L. Tissue engineering a surrogate niche for metastatic cancer cells. Biomaterials 51, 313–319 (2015).
Stiers, P.-J., van Gastel, N., Moermans, K., Stockmans, I. & Carmeliet, G. An ectopic imaging window for intravital imaging of engineered bone tissue. JBMR Plus 2, 92–102 (2018).
Holzapfel, B. M. et al. Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone. Biomaterials 35, 4108–4115 (2014).
Moreau, J. E. et al. Tissue-engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res. 67, 10304–10308 (2007).
Schuster, J., Zhang, J. & Longo, M. A novel human osteoblast-derived severe combined immunodeficiency mouse model of bone metastasis. J. Neurosurg. Spine 4, 388–391 (2006).
Thibaudeau, L. et al. A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. Dis. Models Mech. 7, 299–309 (2014).
Reinisch, A. et al. A humanized bone marrow ossicle xenotransplantation model enables improved engraftment of healthy and leukemic human hematopoietic cells. Nat. Med. 22, 812–821 (2016).
Lee, J. et al. Implantable microenvironments to attract hematopoietic stem/cancer cells. Proc. Natl Acad. Sci. USA 109, 19638–19643 (2012).
Ko, C. Y. et al. The use of chemokine-releasing tissue engineering scaffolds in a model of inflammatory response-mediated melanoma cancer metastasis. Biomaterials 33, 876–885 (2012).
Aguado, B. A. et al. Secretome identification of immune cell factors mediating metastatic cell homing. Sci. Rep. 5, 17566 (2015).
Vaiselbuh, S. R., Edelman, M., Lipton, J. M. & Liu, J. M. Ectopic human mesenchymal stem cell-coated scaffolds in NOD/SCID mice: an in vivo model of the leukemia niche. Tissue Eng. Part C Methods 16, 1523–1531 (2010).
Aguado, B. A., Bushnell, G. G., Rao, S. S., Jeruss, J. S. & Shea, L. D. Engineering the pre-metastatic niche. Nat. Biomed. Eng. 1, 0077 (2017).
Azarin, S. M. et al. In vivo capture and label-free detection of early metastatic cells. Nat. Commun. 6, 8094 (2015).
Rao, S. S. et al. Enhanced survival with implantable scaffolds that capture metastatic breast cancer cells in bivo. Cancer Res. 76, 5209–5218 (2016).
Dvorak, H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 (1986).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015).
Inoue, M. et al. Graft-versus-tumor effect in a patient with advanced neuroblastoma who received HLA haplo-identical bone marrow transplantation. Bone Marrow Transplant. 32, 103–106 (2003).
Lee, J., Shanbhag, S. & Kotov, N. A. Inverted colloidal crystals as three-dimensional microenvironments for cellular co-cultures. J. Mater. Chem. 16, 3558 (2006).
Bryers, J. D., Giachelli, C. M. & Ratner, B. D. Engineering biomaterials to integrate and heal: the biocompatibility paradigm shifts. Biotechnol. Bioeng. 109, 1898–1911 (2012).
Stachowiak, A. N., Bershteyn, A., Tzatzalos, E. & Irvine, D. J. Bioactive hydrogels with an ordered cellular structure combine interconnected macroporosity and robust mechanical properties. Adv. Mater. 17, 399–403 (2005).
Kotov, N. A. et al. Inverted colloidal crystals as three-dimensional cell scaffolds. Langmuir 20, 7887–7892 (2004).
Joao, C. F., Vasconcelos, J. M., Silva, J. C. & Borges, J. P. An overview of inverted colloidal crystal systems for tissue engineering. Tissue Eng. Part B Rev. 20, 437–454 (2014).
Parekkadan, B. & Milwid, J. M. Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 12, 87–117 (2010).
Rutkowski, M. R. et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 27, 27–40 (2015).
Li, A., Dubey, S., Varney, M. L., Dave, B. J. & Singh, R. K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 170, 3369–3376 (2003).
Lee, E. et al. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat. Commun. 5, 4715 (2014).
Havens, A. M. et al. An in vivo mouse model for human prostate cancer metastasis. Neoplasia 10, 371–380 (2008).
Magnon, C. et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361 (2013).
Tai, S. et al. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 71, 1668–1679 (2011).
Kitamura, T., Qian, B. Z. & Pollard, J. W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 15, 73–86 (2015).
Nakahara, T., Norberg, S. M., Shalinsky, D. R., Hu-Lowe, D. D. & McDonald, D. M. Effect of inhibition of vascular endothelial growth factor signaling on distribution of extravasated antibodies in tumors. Cancer Res. 66, 1434–1445 (2006).
Levine, J. E. Implications of TNF-alpha in the pathogenesis and management of GVHD. Int. J. Hematol. 93, 571–577 (2011).
Zeiser, R. & Blazar, B. R. Acute graft-versus-host disease—biologic process, prevention, and therapy. N. Engl. J. Med. 377, 2167–2179 (2017).
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768 (2008).
McFarlane, S. et al. CD44 increases the efficiency of distant metastasis of breast cancer. Oncotarget 6, 11465–11476 (2015).
Taichman, R. S. et al. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS ONE 8, e61873 (2013).
Kobayashi, A. et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J. Exp. Med. 209, 639–639 (2012).
Gao, H. et al. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell 150, 764–779 (2012).
Bragado, P. et al. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat. Cell Biol. 15, 1351–1361 (2013).
Sosa, M. S. et al. NR2F1 controls tumour cell dormancy via SOX9- and RARbeta-driven quiescence programmes. Nat. Commun. 6, 6170 (2015).
Aguirre-Ghiso, J. A., Estrada, Y., Liu, D. & Ossowski, L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res. 63, 1684–1695 (2003).
Yumoto, K. et al. Axl is required for TGF-beta2-induced dormancy of prostate cancer cells in the bone marrow. Sci. Rep. 6, 36520 (2016).
Ruppender, N. et al. Cellular adhesion promotes prostate cancer cells escape from dormancy. PLoS ONE 10, e0130565 (2015).
Novak, M. L. & Koh, T. J. Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 183, 1352–1363 (2013).
McCracken, J. M. & Allen, L. A. Regulation of human neutrophil apoptosis and lifespan in health and disease. J. Cell Death 7, 15–23 (2014).
Hosseini, H. et al. Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 (2016).
Rothwell, P. M. et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 379, 1591–1601 (2012).
Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Jacob, L., Kostev, K., Rathmann, W. & Kalder, M. Impact of metformin on metastases in patients with breast cancer and type 2 diabetes. J. Diabetes Complications 30, 1056–1059 (2016).
Yakes, F. M. et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 10, 2298–2308 (2011).
Dondossola, E. et al. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat. Biomed. Eng. 1, 0007 (2016).
Nair, A. & Tang, L. Influence of scaffold design on host immune and stem cell responses. Semin. Immunol. 29, 62–71 (2017).
Ali, O. A., Huebsch, N., Cao, L., Dranoff, G. & Mooney, D. J. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 8, 151–158 (2009).
Bencherif, S. A. et al. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun. 6, 7556 (2015).
Sadtler, K. et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 352, 366–370 (2016).
Veiseh, O. et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 14, 643–651 (2015).
Swartzlander, M. D. et al. Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels. Biomaterials 41, 79–88 (2015).
Lee, J., Heckl, D. & Parekkadan, B. Multiple genetically engineered humanized microenvironments in a single mouse. Biomater. Res. 20, 19 (2016).
Tentler, J. J. et al. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 9, 338–350 (2012).
White, L., Meyer, P. R. & Benedict, W. F. Establishment and characterization of a human T-cell leukemia line (LALW-2) in nude mice. J. Natl Cancer Inst. 72, 1029–1038 (1984).
Baron, F. et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J. Clin. Oncol. 23, 1993–2003 (2005).
Korngold, R., Marini, J. C., de Baca, M. E., Murphy, G. F. & Giles-Komar, J. Role of tumor necrosis factor-α in graft-versus-host disease and graft-versus-leukemia responses. Biol. Blood Marrow Transplant. 9, 292–303 (2003).
Roth, M. D. & Harui, A. Human tumor infiltrating lymphocytes cooperatively regulate prostate tumor growth in a humanized mouse model. J. Immunother. Cancer 3, 12 (2015).
Simpson-Abelson, M. R. et al. Long-term engraftment and expansion of tumor-derived memory T cells following the implantation of non-disrupted pieces of human lung tumor into NOD-scid IL2R null mice. J. Immunol. 180, 7009–7018 (2008).
El Rayes, T. et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc. Natl Acad. Sci. USA 112, 16000–16005 (2015).
De Cock, J. M. et al. Inflammation triggers Zeb1-dependent escape from tumor latency. Cancer Res. 76, 6778–6784 (2016).
Park, J. et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl Med. 8, 361ra138 (2016).
Wculek, S. K. & Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015).
Engblom, C. et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils. Science 358, eaal5081 (2017).
Rahbari, N. N. et al. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci. Transl Med. 8, 360ra135 (2016).
Sainson, R. C. et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood 111, 4997–5007 (2008).
Murgai, M. et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat. Med. 23, 1176–1190 (2017).
Coffelt, S. B., Wellenstein, M. D. & de Visser, K. E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer 16, 431–446 (2016).
Jespersen, H. et al. Clinical responses to adoptive T-cell transfer can be modeled in an autologous immune-humanized mouse model. Nat. Commun. 8, 707 (2017).
Marin Navarro, A., Susanto, E., Falk, A. & Wilhelm, M. Modeling cancer using patient-derived induced pluripotent stem cells to understand development of childhood malignancies. Cell Death Discov. 4, 7 (2018).
Patel, A. A. et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 214, 1913–1923 (2017).
Drake, A. C., Chen, Q. & Chen, J. Engineering humanized mice for improved hematopoietic reconstitution. Cell. Mol. Immunol. 9, 215–224 (2012).
Powell, D. R. & Huttenlocher, A. Neutrophils in the tumor microenvironment. Trends Immunol. 37, 41–52 (2016).
Reichman, H., Karo-Atar, D. & Munitz, A. Emerging roles for eosinophils in the tumor microenvironment. Trends Cancer 2, 664–675 (2016).
Qian, B. Z. & Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010).
Green, J. J. & Elisseeff, J. H. Mimicking biological functionality with polymers for biomedical applications. Nature 540, 386–394 (2016).
Aguado, B. A. et al. Biomaterial scaffolds as pre-metastatic niche mimics systemically alter the primary tumor and tumor microenvironment. Adv. Healthc. Mater. 7, e1700903 (2018).
MacKie, R. M., Reid, R. & Junor, B. Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. N. Engl. J. Med. 348, 567–568 (2003).
Strauss, D. C. & Thomas, J. M. Transmission of donor melanoma by organ transplantation. Lancet Oncol. 11, 790–796 (2010).
Acknowledgements
We thank the University of Massachusetts Amherst Animal Care Services, A. Burnside for assistance with animal imaging and flow cytometry, and J. Bergan for assistance with tissue clearing and imaging analyses. We also thank J. Chambers and the University of Massachusetts Amherst Light Microscope Facility and Nikon Center of Excellence for assistance with Nikon software and workstations, and L. Minter for discussing human immune cell activity in NSG mice. R.A.C. was supported by a National Science Foundation Research Traineeship (1545399). This work was supported by the National Cancer Institute (R00 CA163671) and the Institute for Applied Life Sciences to J.L.
Author information
Authors and Affiliations
Contributions
R.A.C. and J.L. designed and performed the experiments, analysed and interpreted the results, and wrote the manuscript. J.K. assisted with experiments, conducted image processing and analyses, and participated in the writing of the manuscript. S.R.P. designed the experiments, interpreted the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary Methods 1–3, Supplementary Figures 1–21, Supplementary Table 1 and Supplementary Video Caption 1.
Supplementary Video 1
Tissue-cleared scaffold with blood-vessel and tumour staining.
Rights and permissions
About this article
Cite this article
Carpenter, R.A., Kwak, JG., Peyton, S.R. et al. Implantable pre-metastatic niches for the study of the microenvironmental regulation of disseminated human tumour cells. Nat Biomed Eng 2, 915–929 (2018). https://doi.org/10.1038/s41551-018-0307-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0307-x
This article is cited by
-
How much do we know about the metastatic process?
Clinical & Experimental Metastasis (2024)
-
A synthetic metastatic niche reveals antitumor neutrophils drive breast cancer metastatic dormancy in the lungs
Nature Communications (2023)
-
Fibroblasts in cancer dormancy: foe or friend?
Cancer Cell International (2021)
-
Breast cancer dormancy: need for clinically relevant models to address current gaps in knowledge
npj Breast Cancer (2021)
-
A humanized orthotopic tumor microenvironment alters the bone metastatic tropism of prostate cancer cells
Communications Biology (2021)