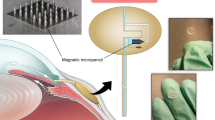
Abstract
Pressures in the intracranial, intraocular and intravascular spaces are clinically useful for the diagnosis and management of traumatic brain injury, glaucoma and hypertension, respectively. Conventional devices for measuring these pressures require surgical extraction after a relevant operational time frame. Bioresorbable sensors, by contrast, eliminate this requirement, thereby minimizing the risk of infection, decreasing the costs of care and reducing distress and pain for the patient. However, the operational lifetimes of bioresorbable pressure sensors available at present fall short of many clinical needs. Here, we present materials, device structures and fabrication procedures for bioresorbable pressure sensors with lifetimes exceeding those of previous reports by at least tenfold. We demonstrate measurement accuracies that compare favourably to those of the most sophisticated clinical standards for non-resorbable devices by monitoring intracranial pressures in rats for 25 days. Assessments of the biodistribution of the constituent materials, complete blood counts, blood chemistry and magnetic resonance imaging compatibility confirm the biodegradability and clinical utility of the device. Our findings establish routes for the design and fabrication of bioresorbable pressure monitors that meet requirements for clinical use.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available in the paper and its Supplementary Information.
References
Jiang, G. Design challenges of implantable pressure monitoring system. Front. Neurosci. 4, 29 (2010).
Yu, L., Kim, B. & Meng, E. Chronically implanted pressure sensors: challenges and state of the field. Sensors 14, 20620–20644 (2014).
Sit, A. J. Continuous monitoring of intraocular pressure: rationale and progress toward a clinical device. J. Glaucoma 18, 272–279 (2009).
Boutry, C. M. et al. Towards biodegradable wireless implants. Phil. Trans. R. Soc. A 370, 2418–2432 (2012).
Chamis, A. L. et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 104, 1029–1033 (2001).
Hall-Stoodley, L., Costerton, J. W. & Stoodley, P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2, 95–108 (2004).
Polikov, V. S., Tresco, P. A. & Reichert, W. M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 (2005).
Kang, S.-K. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016).
Luo, M., Martinez, A. W., Song, C., Herrault, F. & Allen, M. G. A microfabricated wireless RF pressure sensor made completely of biodegradable materials. J. Microelectromech. Syst. 23, 4–13 (2014).
Hwang, S.-W. et al. Biodegradable elastomers and silicon nanomembranes/nanoribbons for stretchable, transient electronics, and biosensors. Nano Lett. 15, 2801–2808 (2015).
Yu, K. J. et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 15, 782–791 (2016).
Lee, Y. K. et al. Dissolution of monocrystalline silicon nanomembranes and their use as encapsulation layers and electrical interfaces in water-soluble electronics. ACS Nano 11, 12562–12572 (2017).
Lee, G. et al. Fully biodegradable microsupercapacitor for power storage in transient electronics. Adv. Energy Mater. 7, 1700157 (2017).
Lee, C. H. et al. Wireless microfluidic systems for programmed, functional transformation of transient electronic devices. Adv. Funct. Mater. 25, 5100–5106 (2015).
Tao, H. et al. Silk-based resorbable electronic devices for remotely controlled therapy and in vivo infection abatement. Proc. Natl Acad. Sci. USA 111, 17385–17389 (2014).
Hwang, S.-W. et al. A physically transient form of silicon electronics. Science 337, 1640–1644 (2012).
Hwang, S.-W. et al. High-performance biodegradable/transient electronics on biodegradable polymers. Adv. Mater. 26, 3905–3911 (2014).
Kang, S.-K. et al. Dissolution behaviors and applications of silicon oxides and nitrides in transient electronics. Adv. Funct. Mater. 24, 4427–4434 (2014).
Kang, S.-K. et al. Biodegradable thin metal foils and spin-on glass materials for transient electronics. Adv. Funct. Mater. 25, 1789–1797 (2015).
Fang, H. et al. Ultrathin, transferred layers of thermally grown silicon dioxide as biofluid barriers for biointegrated flexible electronic systems. Proc. Natl Acad. Sci. USA 113, 11682–11687 (2016).
Lee, Y. K. et al. Kinetics and chemistry of hydrolysis of ultrathin, thermally grown layers of silicon oxide as biofluid barriers in flexible electronic systems. ACS Appl. Mater. Interfaces 9, 42633–42638 (2017).
Haddad, S. H. & Arabi, Y. M. Critical care management of severe traumatic brain injury in adults. Scand. J. Trauma Resusc. Emerg. Med. 20, 12 (2012).
Wang, Q., Ding, J. & Wang, W. Fabrication and temperature coefficient compensation technology of low cost high temperature pressure sensor. Sens. Actuat. A 120, 468–473 (2005).
Camino, G., Lomakin, S. M. & Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer 42, 2395–2402 (2001).
Kanda, Y. A graphical representation of the piezoresistance coefficients in silicon. IEEE Trans. Electron Devices 29, 64–70 (1982).
Lund, E. & Finstad, T. G. Temperature and doping dependency of piezoresistivity in p-type silicon. MRS Proc. 657, EE5.13 (2000).
Norton, P. & Brandt, J. Temperature coefficient of resistance for p- and n-type silicon. Solid-State Electron. 21, 969–974 (1978).
Bratton, S. L. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J. Neurotrauma 24, S37–S44 (2007).
Post Craniotomy Subdural Pressure Monitoring Kit. Model 110-4G (Integra NeuroSciences, 2010).
Johanson, C. E. et al. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid. Res. 5, 10 (2008).
Moghimi, S. M. & Patel, H. M. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system – the concept of tissue specificity. Adv. Drug Deliv. Rev. 32, 45–60 (1998).
Gelabert-González, M. et al. The Camino® intracranial pressure device in clinical practice. Assessment in a 1000 cases. Acta Neurochir. 148, 435–441 (2006).
Martínez-Mañas, R. M., Santamarta, D., de Campos, J. M. & Ferrer, E. Camino intracranial pressure monitor: prospective study of accuracy and complications. J. Neurol. Neurosurg. Psychiat. 69, 82–86 (2000).
Zacchetti, L., Magnoni, S., Di Corte, F., Zanier, E. R. & Stocchetti, N. Accuracy of intracranial pressure monitoring: systematic review and meta-analysis. Crit. Care 19, 420 (2015).
Dunn, J. F. et al. Functional mapping at 9.4T using a new MRI compatible electrode chronically implanted in rats. Magn. Reson. Med. 61, 222–228 (2009).
Sawyer-Glover, A. M. & Shellock, F. G. Pre-MRI procedure screening: recommendations and safety considerations for biomedical implants and devices. J. Magn. Reson. Imaging 12, 92–106 (2000).
National Research Council Guide for the Care and Use of Laboratory Animals 8th edn (The National Academies Press, 2011).
Acknowledgements
J.S. thanks G. Mensing and J. Maduzia at the Micro-Nano-Mechanical Systems Cleanroom (University of Illinois at Urbana–Champaign) for assistance with process development. J.S. acknowledges support from True Phantom Solutions Inc. with the preparation of brain phantoms for MRI compatibility tests. Y.X. acknowledges support from the Ryan Fellowship and the Northwestern University International Institute for Nanotechnology. L.T. acknowledges support from a Beckman Institute Postdoctoral Fellowship at UIUC. I.K. acknowledges support from Cancer Center Support grant no. P30 CA060553 (National Cancer Institute) awarded to the Robert H. Lurie Comprehensive Cancer Center. K.J.Y. acknowledges support from the National Research Foundation of Korea (grant nos. NRF-2017M1A2A2048880 and NRF-2018M3A7B4071109) and Yonsei University Future-leading Research Initiative of 2017 (grant no. RMS2 2018-22-0028). Y.H. acknowledges support from the NSF (grant nos. 1400169, 1534120 and 1635443).
Author information
Authors and Affiliations
Contributions
J.S., W.B., Y.L., M.C., J.K., H.R., J.-K.C., S.-K.K., S.M.W., K.J.Y. and J.A.R. designed and fabricated the sensors. J.S., Y.L., M.C., H.R., J.Z. and Y.K.L. conceived and performed the dissolution studies. J.S., W.B., I.K. and M.P. performed the biodistribution, toxicity and histology studies. Y.Y., P.G., M.R.M. and W.Z.R. performed the in vivo ICP measurements and analysed data. Y.X. and Y.H. performed mechanical simulations. J.S., Y.Y., L.T., C.R.H., W.S. and S.-K.S. performed the CT and MRI studies. J.S., Y.Y., W.B., Y.X., W.Z.R. and J.A.R. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary notes, references and figures.
Rights and permissions
About this article
Cite this article
Shin, J., Yan, Y., Bai, W. et al. Bioresorbable pressure sensors protected with thermally grown silicon dioxide for the monitoring of chronic diseases and healing processes. Nat Biomed Eng 3, 37–46 (2019). https://doi.org/10.1038/s41551-018-0300-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0300-4
This article is cited by
-
Fully bioresorbable hybrid opto-electronic neural implant system for simultaneous electrophysiological recording and optogenetic stimulation
Nature Communications (2024)
-
Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring
Nano-Micro Letters (2024)
-
Intracranial pressure monitoring in neurosurgery: the present situation and prospects
Chinese Neurosurgical Journal (2023)
-
Frictionless multiphasic interface for near-ideal aero-elastic pressure sensing
Nature Materials (2023)
-
Zinc hybrid sintering for printed transient sensors and wireless electronics
npj Flexible Electronics (2023)