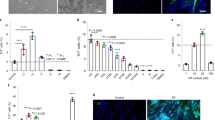
Abstract
Adult skeletal muscle has a robust capacity for self-repair, owing to synergies between muscle satellite cells and the immune system. In vitro models of muscle self-repair would facilitate the basic understanding of muscle regeneration and the screening of therapies for muscle disease. Here, we show that the incorporation of macrophages into muscle tissues engineered from adult-rat myogenic cells enables near-complete structural and functional repair after cardiotoxic injury in vitro. First, we show that—in contrast with injured neonatal-derived engineered muscle—adult-derived engineered muscle fails to properly self-repair after injury, even when treated with pro-regenerative cytokines. We then show that rat bone-marrow-derived macrophages or human blood-derived macrophages resident within the in vitro engineered tissues stimulate muscle satellite cell-mediated myogenesis while significantly limiting myofibre apoptosis and degeneration. Moreover, bone-marrow-derived macrophages within engineered tissues implanted in a mouse dorsal window-chamber model augmented blood vessel ingrowth, cell survival, muscle regeneration and contractile function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others

Data availability
All the data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Charge, S. B. & Rudnicki, M. A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84, 209–238 (2004).
Lepper, C., Partridge, T. A. & Fan, C. M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646 (2011).
Yin, H., Price, F. & Rudnicki, M. A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 93, 23–67 (2013).
Tidball, J. G. & Villalta, S. A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1173–R1187 (2010).
Kharraz, Y., Guerra, J., Mann, C. J., Serrano, A. L. & Munoz-Canoves, P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators Inflamm. 2013, 491497 (2013).
Saclier, M. et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 31, 384–396 (2013).
Tidball, J. G., Dorshkind, K. & Wehling-Henricks, M. Shared signaling systems in myeloid cell-mediated muscle regeneration. Development 141, 1184–1196 (2014).
Turner, N. J. & Badylak, S. F. Regeneration of skeletal muscle. Cell Tissue Res. 347, 759–774 (2012).
Blau, H. M., Cosgrove, B. D. & Ho, A. T. The central role of muscle stem cells in regenerative failure with aging. Nat. Med. 21, 854–862 (2015).
Sacco, A. et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell 143, 1059–1071 (2010).
Bhatia, S. N. & Ingber, D. E. Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014).
Clevers, H. Modeling development and disease with organoids. Cell 165, 1586–1597 (2016).
Quarta, M. et al. An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat. Biotechnol. 34, 752–759 (2016).
Cosgrove, B. D. et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 20, 255–264 (2014).
Gilbert, P. M. et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078–1081 (2010).
Juhas, M., Engelmayr, G. C. Jr, Fontanella, A. N., Palmer, G. M. & Bursac, N. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proc. Natl Acad. Sci. USA 111, 5508–5513 (2014).
Juhas, M. & Bursac, N. Roles of adherent myogenic cells and dynamic culture in engineered muscle function and maintenance of satellite cells. Biomaterials 35, 9438–9446 (2014).
Close, R. Dynamic properties of fast and slow skeletal muscles of rat during development. J. Physiol. 173, 74–95 (1964).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Madden, L., Juhas, M., Kraus, W. E., Truskey, G. A. & Bursac, N. Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs. eLife 4, e04885 (2015).
Salva, M. Z. et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol. Ther. 15, 320–329 (2007).
Pelosi, L. et al. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 21, 1393–1402 (2007).
Deng, B., Wehling-Henricks, M., Villalta, S. A., Wang, Y. & Tidball, J. G. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 189, 3669–3680 (2012).
Hayashiji, N. et al. G-CSF supports long-term muscle regeneration in mouse models of muscular dystrophy. Nat. Commun. 6, 6745 (2015).
Schleicher, U. & Bogdan, C. Generation, culture and flow-cytometric characterization of primary mouse macrophages. Methods Mol. Biol. 531, 203–224 (2009).
Hamilton, J. A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8, 533–544 (2008).
Fleetwood, A. J., Lawrence, T., Hamilton, J. A. & Cook, A. D. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J. Immunol. 178, 5245–5252 (2007).
Krause, M. P. et al. Impaired macrophage and satellite cell infiltration occurs in a muscle-specific fashion following injury in diabetic skeletal muscle. PLoS ONE 8, e70971 (2013).
Soleimani, M. & Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 4, 102–106 (2009).
Dirks, A. & Leeuwenburgh, C. Apoptosis in skeletal muscle with aging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R519–R527 (2002).
Stratos, I. et al. Inhibition of caspase mediated apoptosis restores muscle function after crush injury in rat skeletal muscle. Apoptosis 17, 269–277 (2012).
Wang, H. et al. Turning terminally differentiated skeletal muscle cells into regenerative progenitors. Nat. Commun. 6, 7916 (2015).
Fernando, P., Kelly, J. F., Balazsi, K., Slack, R. S. & Megeney, L. A. Caspase 3 activity is required for skeletal muscle differentiation. Proc. Natl Acad. Sci. USA 99, 11025–11030 (2002).
Chazaud, B. et al. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell. Biol. 163, 1133–1143 (2003).
Sonnet, C. et al. Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. J. Cell. Sci. 119, 2497–2507 (2006).
Kondo, M. et al. Roles of proinflammatory cytokines and the Fas/Fas ligand interaction in the pathogenesis of inflammatory myopathies. Immunology 128, e589–e599 (2009).
Kalovidouris, A. E. & Plotkin, Z. Synergistic cytotoxic effect of interferon-gamma and tumor necrosis factor-alpha on cultured human muscle cells. J. Rheumatol. 22, 1698–1703 (1995).
Reid, M. B. & Li, Y. P. Tumor necrosis factor-alpha and muscle wasting: a cellular perspective. Respir. Res. 2, 269–272 (2001).
Pistilli, E. E., Jackson, J. R. & Alway, S. E. Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis 11, 2115–2126 (2006).
Geng, Y. J., Wu, Q., Muszynski, M., Hansson, G. K. & Libby, P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-gamma, tumor necrosis factor-alpha, and interleukin-1 beta. Arterioscler. Thromb. Vasc. Biol. 16, 19–27 (1996).
Pedersen, B. K. Exercise-induced myokines and their role in chronic diseases. Brain Behav. Immun. 25, 811–816 (2011).
Pedersen, B. K., Steensberg, A. & Schjerling, P. Muscle-derived interleukin-6: possible biological effects. J. Physiol. 536, 329–337 (2001).
Rehman, J. et al. Dynamic exercise leads to an increase in circulating ICAM-1: further evidence for adrenergic modulation of cell adhesion. Brain Behav. Immun. 11, 343–351 (1997).
Villalta, S. A. et al. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum. Mol. Genet. 20, 790–805 (2011).
Zeng, L. et al. Insulin-like 6 is induced by muscle injury and functions as a regenerative factor. J. Biol. Chem. 285, 36060–36069 (2010).
Munoz-Canoves, P., Scheele, C., Pedersen, B. K. & Serrano, A. L. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 280, 4131–4148 (2013).
Jablonski, K. A. et al. Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE 10, e0145342 (2015).
Wajant, H., Pfizenmaier, K. & Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 10, 45–65 (2003).
Grounds, M. D. & Torrisi, J. Anti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J. 18, 676–682 (2004).
He, M. M. et al. Small-molecule inhibition of TNF-alpha. Science 310, 1022–1025 (2005).
Chen, S. E. et al. Role of TNF-α signaling in regeneration of cardiotoxin-injured muscle. Am. J. Physiol. Cell Physiol. 289, C1179–C1187 (2005).
Cheng, M., Nguyen, M. H., Fantuzzi, G. & Koh, T. J. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 294, C1183–C1191 (2008).
Montarras, D. et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science 309, 2064–2067 (2005).
Day, K., Shefer, G., Shearer, A. & Yablonka-Reuveni, Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev. Biol. 340, 330–343 (2010).
Tierney, M. T. et al. Autonomous extracellular matrix remodeling controls a progressive adaptation in muscle stem cell regenerative capacity during development. Cell Rep. 14, 1940–1952 (2016).
Lees, S. J., Zwetsloot, K. A. & Booth, F. W. Muscle precursor cells isolated from aged rats exhibit an increased tumor necrosis factor-alpha response. Aging Cell 8, 26–35 (2009).
Fulle, S., Sancilio, S., Mancinelli, R., Gatta, V. & Di Pietro, R. Dual role of the caspase enzymes in satellite cells from aged and young subjects. Cell Death Dis. 4, e955 (2013).
Davis, T. A. & Fiorotto, M. L. Regulation of muscle growth in neonates. Curr. Opin. Clin. Nutr. 12, 78–85 (2009).
Dogra, C., Changotra, H., Mohan, S. & Kumar, A. Tumor necrosis factor-like weak inducer of apoptosis inhibits skeletal myogenesis through sustained activation of nuclear factor-κB and degradation of MyoD protein. J. Biol. Chem. 281, 10327–10336 (2006).
Bruunsgaard, H., Pedersen, M. & Pedersen, B. K. Aging and proinflammatory cytokines. Curr. Opin. Hematol. 8, 131–136 (2001).
Collins, R. A. & Grounds, M. D. The role of tumor necrosis factor-α (TNF-α) in skeletal muscle regeneration. Studies in TNF-α-/- and TNF-α-/-/LT-α-/- mice. J. Histochem. Cytochem. 49, 989–1001 (2001).
Ochoa, O. et al. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R651–R661 (2007).
Chung, E. S. et al. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am. J. Pathol. 175, 1984–1992 (2009).
Nucera, S., Biziato, D. & De Palma, M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int. J. Dev. Biol. 55, 495–503 (2011).
Vignaud, A. et al. Impaired skeletal muscle repair after ischemia–reperfusion injury in mice. J. Biomed. Biotechnol. 2010, 724914 (2010).
Lee, S. L., Pevec, W. C. & Carlsen, R. C. Functional outcome of new blood vessel growth into ischemic skeletal muscle. J. Vasc. Surg. 34, 1096–1102 (2001).
Davies, L. C., Jenkins, S. J., Allen, J. E. & Taylor, P. R. Tissue-resident macrophages. Nat. Immunol. 14, 986–995 (2013).
Mantovani, A., Biswas, S. K., Galdiero, M. R., Sica, A. & Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229, 176–185 (2013).
Lavin, Y. et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014).
Das, A. et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 185, 2596–2606 (2015).
Forbes, S. J. & Rosenthal, N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat. Med. 20, 857–869 (2014).
Aurora, A. B. et al. Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 124, 1382–1392 (2014).
Kanters, E. et al. Hematopoietic NF-kappaB1 deficiency results in small atherosclerotic lesions with an inflammatory phenotype. Blood 103, 934–940 (2004).
Lesault, P. F. et al. Macrophages improve survival, proliferation and migration of engrafted myogenic precursor cells into MDX skeletal muscle. PLoS ONE 7, e46698 (2012).
Stanley, E. R. Murine bone marrow-derived macrophages. Methods Mol. Biol. 75, 301–304 (1997).
Gleissner, C. A., Shaked, I., Little, K. M. & Ley, K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J. Immunol. 184, 4810–4818 (2010).
Flick, D. A. & Gifford, G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J. Immunol. Methods 68, 167–175 (1984).
Liddil, J. D., Dorr, R. T. & Scuderi, P. Association of lysosomal activity with sensitivity and resistance to tumor necrosis factor in murine L929 cells. Cancer Res. 49, 2722–2728 (1989).
Bellucci, J. J., Amiram, M., Bhattacharyya, J., McCafferty, D. & Chilkoti, A. Three-in-one chromatography-free purification, tag removal, and site-specific modification of recombinant fusion proteins using sortase A and elastin-like polypeptides. Angew. Chem. Int. Ed. Engl. 52, 3703–3708 (2013).
Jetten, N. et al. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17, 109–118 (2014).
Jackman, C. P., Carlson, A. L. & Bursac, N. Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials. 111, 66–79 (2016).
Hinds, S., Bian, W., Dennis, R. G. & Bursac, N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 32, 3575–3583 (2011).
Ma, L. et al. A novel small-molecule tumor necrosis factor alpha inhibitor attenuates inflammation in a hepatitis mouse model. J. Biol. Chem. 289, 12457–12466 (2014).
Palmer, G. M. et al. In vivo optical molecular imaging and analysis in mice using dorsal window chamber models applied to hypoxia, vasculature and fluorescent reporters. Nat. Protoc. 6, 1355–1366 (2011).
Palmer, G. M., Fontanella, A. N., Shan, S. & Dewhirst, M. W. High-resolution in vivo imaging of fluorescent proteins using window chamber models. Methods Mol. Biol. 872, 31–50 (2012).
Acknowledgements
We acknowledge C. Jackman, A. Khodabukus, L. Li, I. Shadrin, A. Ganapathi, G. Palmer and G. Hanna for technical assistance, and the Light Microscopy and Optical Molecular Imaging and Analysis core facilities at Duke University for use of their resources. We also thank B. Chazaud for granting a protocol for human macrophage derivation. This study was supported by the National Science Foundation’s Graduate Research Fellowship to M.J., and grants AR070543 and AR065873 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to N.B.
Author information
Authors and Affiliations
Contributions
M.J. and N.B. conceived and designed the research. M.J., N.A., J.T.W., J.Y., Z.S. and C.S. performed the experiments. Y.Q. performed the implantation surgery. M.J., N.A., J.Y., Z.S. and N.B. analysed the results. M.J. and N.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Supplementary Video 1
Real-time assessment of engineered muscle regeneration in vitro.
Supplementary Video 2
Perfused ingrown blood vessels within engineered muscle implants.
Supplementary Video 3
In vivo spontaneous calcium transients in engineered muscle implants.
Supplementary Video 4
Ex vivo electrically induced calcium transients in engineered muscle explants.
Rights and permissions
About this article
Cite this article
Juhas, M., Abutaleb, N., Wang, J.T. et al. Incorporation of macrophages into engineered skeletal muscle enables enhanced muscle regeneration. Nat Biomed Eng 2, 942–954 (2018). https://doi.org/10.1038/s41551-018-0290-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0290-2
This article is cited by
-
Elastic porous microspheres/extracellular matrix hydrogel injectable composites releasing dual bio-factors enable tissue regeneration
Nature Communications (2024)
-
Skeletal muscle regeneration failure in ischemic-damaged limbs is associated with pro-inflammatory macrophages and premature differentiation of satellite cells
Genome Medicine (2023)
-
USP18 is an essential regulator of muscle cell differentiation and maturation
Cell Death & Disease (2023)
-
FNIP1 abrogation promotes functional revascularization of ischemic skeletal muscle by driving macrophage recruitment
Nature Communications (2023)
-
Mechanisms of cooperative cell-cell interactions in skeletal muscle regeneration
Inflammation and Regeneration (2022)