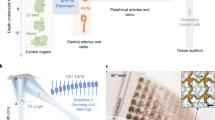
Abstract
Continuous monitoring of the central blood pressure waveform from deeply embedded vessels such as the carotid artery and jugular vein has clinical value for the prediction of all-cause cardiovascular mortality. However, existing non-invasive approaches, including photoplethysmography and tonometry, only enable access to the superficial peripheral vasculature. Although current ultrasonic technologies allow non-invasive deep tissue observation, unstable coupling with the tissue surface resulting from the bulkiness and rigidity of conventional ultrasound probes introduces usability constraints. Here, we describe the design and operation of an ultrasonic device that is conformal to the skin and capable of capturing blood pressure waveforms at deeply embedded arterial and venous sites. The wearable device is ultrathin (240 μm) and stretchable (with strains up to 60%), and enables the non-invasive, continuous and accurate monitoring of cardiovascular events from multiple body locations, which should facilitate its use in a variety of clinical environments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the Article and its Supplementary Information. The raw data generated in this study are available from the corresponding author upon reasonable request.
References
McGhee, B. H. & Bridges, E. J. Monitoring arterial blood pressure: what you may not know. Crit. Care Nurse 22, 60–79 (2002).
Avolio, A. P., Butlin, M. & Walsh, A. Arterial blood pressure measurement and pulse wave analysis—their role in enhancing cardiovascular assessment. Physiol. Meas. 31, 275–290 (2009).
Kumar, A. et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit. Care Med. 32, 691–699 (2004).
Dagdeviren, C. et al. Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nat. Commun. 5, 4496–4506 (2014).
Safar, M. E. et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension 39, 735–738 (2002).
Trudeau, L. Central blood pressure as an index of antihypertensive control: determinants and potential value. Can. J. Cardiol. 30, 23–28 (2014).
McEniery, C. M., Cockcroft, J. R., Roman, M. J., Franklin, S. S. & Wilkinson, I. B. Central blood pressure: current evidence and clinical importance. Eur. Heart J. 35, 1719–1725 (2014).
Ding, F.-H. et al. Validation of the noninvasive assessment of central blood pressure by the SphygmoCor and Omron devices against the invasive catheter measurement. Am. J. Hypertens. 24, 1306–1311 (2011).
Agabiti-Rosei, E. et al. Central blood pressure measurements and antihypertensive therapy. Hypertension 50, 154–160 (2007).
Bruyndonckx, L. et al. Methodological considerations and practical recommendations for the application of peripheral arterial tonometry in children and adolescents. Int. J. Cardiol. 168, 3183–3190 (2013).
Avolio, A. P. et al. Role of pulse pressure amplification in arterial hypertension. Hypertension 54, 375–383 (2009).
Williams, B. et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes. Circulation 113, 1213–1225 (2006).
Moffitt, E. A. et al. Rate–pressure product correlates poorly with myocardial oxygen consumption during anaesthesia in coronary patients. Can. J. Anaesth. 31, 5–12 (1984).
Roman, M. J. et al. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart Study. J. Hypertens. 28, 384–388 (2010).
Chen, C.-H. et al. Different effects of fosinopril and atenolol on wave reflections in hypertensive patients. Hypertension 25, 1034–1041 (1995).
Pini, R. et al. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J. Am. Coll. Cardiol. 51, 2432–2439 (2008).
Langewouters, G., Settels, J., Roelandt, R. & Wesseling, K. Why use Finapres or Portapres rather than intraarterial or intermittent non-invasive techniques of blood pressure measurement? J. Med. Eng. Technol. 22, 37–43 (1998).
Kim, J. et al. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2, e1600418 (2016).
Schwartz, G. et al. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 4, 1859–1870 (2013).
Sandberg, M., Zhang, Q., Styf, J., Gerdle, B. & Lindberg, L. G. Non‐invasive monitoring of muscle blood perfusion by photoplethysmography: evaluation of a new application. Acta Physiol. 183, 335–343 (2005).
Hertzman, A. B. The blood supply of various skin areas as estimated by the photoelectric plethysmograph. Am. J. Physiol. Cell. Physiol. 124, 328–340 (1938).
Maeda, Y., Sekine, M. & Tamura, T. Relationship between measurement site and motion artifacts in wearable reflected photoplethysmography. J. Med. Syst. 35, 969–976 (2011).
Xing, X. & Sun, M. Optical blood pressure estimation with photoplethysmography and FFT-based neural networks. Biomed. Opt. Express 7, 3007–3020 (2016).
Drzewiecki, G. M., Melbin, J. & Noordergraaf, A. Arterial tonometry: review and analysis. J. Biomech. 16, 141–152 (1983).
Howard, G. et al. Carotid artery intimal-medial thickness distribution in general populations as evaluated by B-mode ultrasound. Stroke 24, 1297–1304 (1993).
Rotenberg, M. Y. & Tian, B. Bioelectronic devices: long-lived recordings. Nat. Biomed. Eng. 1, 48–50 (2017).
Kim, D.-H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Yokota, T. et al. Ultraflexible, large-area, physiological temperature sensors for multipoint measurements. Proc. Natl Acad. Sci. USA 112, 14533–14538 (2015).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Martín, A. et al. Epidermal microfluidic electrochemical detection system: enhanced sweat sampling and metabolite detection. ACS Sens. 2, 1860–1868 (2017).
Huang, X. et al. Materials and designs for wireless epidermal sensors of hydration and strain. Adv. Funct. Mater. 24, 3846–3854 (2014).
Arndt, J. O., Klauske, J. & Mersch, F. The diameter of the intact carotid artery in man and its change with pulse pressure. Pflugers Arch. 301, 230–240 (1968).
Zhou, Q., Lam, K. H., Zheng, H., Qiu, W. & Shung, K. K. Piezoelectric single crystal ultrasonic transducers for biomedical applications. Prog. Mater. Sci. 66, 87–111 (2014).
Sun, P. et al. High frequency PMN-PT 1–3 composite transducer for ultrasonic imaging application. Ferroelectrics 408, 120–128 (2010).
Arumugam, V., Naresh, M. & Sanjeevi, R. Effect of strain rate on the fracture behaviour of skin. J. Biosci. 19, 307–313 (1994).
Kitamura, K., Jorgensen, C. R., Gobel, F. L., Taylor, H. L. & Wang, Y. Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J. Appl. Physiol. 32, 516–522 (1972).
Ishibashi, Y., Duncker, D. J., Zhang, J. & Bache, R. J. ATP-sensitive K+ channels, adenosine, and nitric oxide-mediated mechanisms account for coronary vasodilation during exercise. Circ. Res. 82, 346–359 (1998).
Wain, R. A. et al. Accuracy of duplex ultrasound in evaluating carotid artery anatomy before endarterectomy. J. Vasc. Surg. 27, 235–244 (1998).
Donahue, S. P., Wood, J. P., Patel, B. M. & Quinn, J. V. Correlation of sonographic measurements of the internal jugular vein with central venous pressure. Am. J. Emerg. Med. 27, 851–855 (2009).
Butman, S. M., Ewy, G. A., Standen, J. R., Kern, K. B. & Hahn, E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. J. Am. Coll. Cardiol. 22, 968–974 (1993).
Mukkamala, R. & Xu, D. Continuous and less invasive central hemodynamic monitoring by blood pressure waveform analysis. Am. J. Physiol. Heart Circ. Physiol. 299, 584–599 (2010).
Camacho, F., Avolio, A. & Lovell, N. Estimation of pressure pulse amplification between aorta and brachial artery using stepwise multiple regression models. Physiol. Meas. 25, 879–889 (2004).
Williams, B. et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 113, 1213–1225 (2006).
Shirwany, N. A. & Zou, M.-h Arterial stiffness: a brief review. Acta Pharmacol. Sin. 31, 1267–1276 (2010).
DeLoach, S. S. & Townsend, R. R. Vascular stiffness: its measurement and significance for epidemiologic and outcome studies. Clin. J. Am. Soc. Nephrol. 3, 184–192 (2008).
Mukkamala, R. et al. Toward ubiquitous blood pressure monitoring via pulse transit time: theory and practice. IEEE. Trans. Biomed. Eng. 62, 1879–1901 (2015).
Ren, K., Liu, Y., Geng, X., Hofmann, H. F. & Zhang, Q. M. Single crystal PMN-PT/epoxy 1–3 composite for energy-harvesting application. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 53, 631–638 (2006).
Hu, H. et al. Stretchable ultrasonic transducer arrays for three-dimensional imaging on complex surfaces. Sci. Adv. 4, eaar3979 (2018).
Acknowledgements
The project was supported by the National Institutes of Health (NIH, grant R21EB025521) and the Center for Wearable Sensors at the University of California, San Diego. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. All bio-experiments were conducted in accordance with the ethical guidelines of the NIH and with the approval of the Institutional Review Board of the University of California, San Diego (IRB no. 170812). The authors thank K. Anagnostopoulos and H. Kim for discussions and advice regarding Picoscope DAQ, A. Kahn for discussions on PWV measurements, E. Topol, S. Steinhubl and E. Muse for stimulating discussions on ambulatory BP measurement, Q. Yang and R. Lal for mechanical vibration characterization of the 1–3 composite material, and S. Xiang for constructive feedback on manuscript preparation.
Author information
Authors and Affiliations
Contributions
Chonghe Wang and S.X. designed the research. Chonghe Wang, X.L., M.L., Z.Z. and H.H. performed the experiment. Chonghe Wang performed the simulation. Chonghe Wang, M.L., Z.Z. and X.L. analysed the data. Chonghe Wang, Z.Z. and S.X. wrote the paper. All authors provided active and valuable feedback on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary notes, figures, tables and references.
Rights and permissions
About this article
Cite this article
Wang, C., Li, X., Hu, H. et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat Biomed Eng 2, 687–695 (2018). https://doi.org/10.1038/s41551-018-0287-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0287-x
This article is cited by
-
Deep self-supervised machine learning algorithms with a novel feature elimination and selection approaches for blood test-based multi-dimensional health risks classification
BMC Bioinformatics (2024)
-
Wearable ultrasound for continuous deep-tissue monitoring
Nature Biotechnology (2024)
-
Microarrow sensor array with enhanced skin adhesion for transdermal continuous monitoring of glucose and reactive oxygen species
Bio-Design and Manufacturing (2024)
-
Flexible large-area ultrasound arrays for medical applications made using embossed polymer structures
Nature Communications (2024)
-
A fully integrated wearable ultrasound system to monitor deep tissues in moving subjects
Nature Biotechnology (2024)