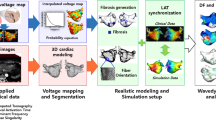
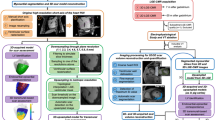
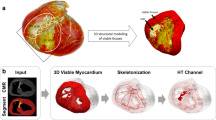
Abstract
Ventricular tachycardia (VT), which can lead to sudden cardiac death, occurs frequently in patients with myocardial infarction. Catheter-based radio-frequency ablation of cardiac tissue has achieved only modest efficacy, owing to the inaccurate identification of ablation targets by current electrical mapping techniques, which can lead to extensive lesions and to a prolonged, poorly tolerated procedure. Here, we show that personalized virtual-heart technology based on cardiac imaging and computational modelling can identify optimal infarct-related VT ablation targets in retrospective animal (five swine) and human studies (21 patients), as well as in a prospective feasibility study (five patients). We first assessed, using retrospective studies (one of which included a proportion of clinical images with artefacts), the capability of the technology to determine the minimum-size ablation targets for eradicating all VTs. In the prospective study, VT sites predicted by the technology were targeted directly, without relying on prior electrical mapping. The approach could improve infarct-related VT ablation guidance, where accurate identification of patient-specific optimal targets could be achieved on a personalized virtual heart before the clinical procedure.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stevenson, W. G. et al. Radiofrequency catheter ablation of ventricular tachycardia after myocardial infarction. Circulation 98, 308–314 (1998).
Aliot, E. M. et al. EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias. Heart Rhythm 6, 886–933 (2009).
Zhong, H., Lacomis, J. M. & Schwartzman, D. On the accuracy of CartoMerge for guiding posterior left atrial ablation in man. Heart Rhythm 4, 595–602 (2007).
Brugada, J. et al. Nonsurgical transthoracic epicardial radiofrequency ablation: an alternative in incessant ventricular tachycardia. J. Am. Coll. Cardiol. 41, 2036–2043 (2003).
Sosa, E., Scanavacca, M., d’Avila, A., Oliveira, F. & Ramires, J. A. Nonsurgical transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. J. Am. Coll. Cardiol. 35, 1442–1449 (2000).
Dong, J. et al. Impact of heart rhythm status on registration accuracy of the left atrium for catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 18, 1269–1276 (2007).
de Bakker, J. M. et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation 77, 589–606 (1988).
Peters, N. S. & Wit, A. L. Myocardial architecture and ventricular arrhythmogenesis. Circulation 97, 1746–1754 (1998).
Callans, D. J. et al. Efficacy of radiofrequency catheter ablation for ventricular tachycardia in healed myocardial infarction. Am J Cardiol 82, 429–432 (1998).
Calkins, H. et al. Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. Cooled RF Multi Center Investigators Group. J. Am. Coll. Cardiol. 35, 1905–1914 (2000).
Arevalo, H. J. et al. Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models. Nat. Commun. 7, 11437 (2016).
Prakosa, A. et al. Methodology for image-based reconstruction of ventricular geometry for patient-specific modeling of cardiac electrophysiology. Prog. Biophys. Mol. Biol. 115, 226–234 (2014).
Bayer, J. D., Blake, R. C., Plank, G. & Trayanova, N. A. A novel rule-based algorithm for assigning myocardial fiber orientation to computational heart models. Ann. Biomed. Eng. 40, 2243–2254 (2012).
Zahid, S. et al. Feasibility of using patient-specific models and the “minimum cut” algorithm to predict optimal ablation targets for left atrial flutter. Heart Rhythm 13, 1687–1698 (2016).
Lardo, A. C. et al. Visualization and temporal/spatial characterization of cardiac radiofrequency ablation lesions using magnetic resonance imaging. Circulation 102, 698–705 (2000).
Tung, R., Josephson, M. E., Reddy, V., Reynolds, M. R. & SMASH-VT Investigators. Influence of clinical and procedural predictors on ventricular tachycardia ablation outcomes: an analysis from the substrate mapping and ablation in Sinus Rhythm to Halt Ventricular Tachycardia Trial (SMASH-VT). J. Cardiovasc. Electrophysiol. 21, 799–803 (2010).
Hsia, H. H., Lin, D., Sauer, W. H., Callans, D. J. & Marchlinski, F. E. Anatomic characterization of endocardial substrate for hemodynamically stable reentrant ventricular tachycardia: identification of endocardial conducting channels. Heart Rhythm 3, 503–512 (2006).
Tung, R. et al. Impact of local ablation on interconnected channels within ventricular scar: mechanistic implications for substrate modification. Circ Arrhythm Electrophysiol 6, 1131–1138 (2013).
Verma, A. et al. Relationship between successful ablation sites and the scar border zone defined by substrate mapping for ventricular tachycardia post-myocardial infarction. J. Cardiovasc. Electrophysiol. 16, 465–471 (2005).
Krummen, D. E. et al. Modifying ventricular fibrillation by targeted rotor substrate ablation: proof-of-concept from experimental studies to clinical VF. J. Cardiovasc. Electrophysiol. 26, 1117–1126 (2015).
Nikolov, P., Prakosa, A., Arevalo, H. J., Wu, K. C. & Trayanova, N. A novel approach to arrhythmia risk stratification in patients with non-ischemic cardiomyopathy. Circulation 134, A20903–A20903 (2016).
Estner, H. L. et al. The critical isthmus sites of ischemic ventricular tachycardia are in zones of tissue heterogeneity, visualized by magnetic resonance imaging. Heart Rhythm 8, 1942–1949 (2011).
Ashikaga, H. et al. Feasibility of image-based simulation to estimate ablation target in human ventricular arrhythmia. Heart Rhythm 10, 1109–1116 (2013).
Schmidt, A. et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 115, 2006–2014 (2007).
Prassl, A. J. et al. Automatically generated, anatomically accurate meshes for cardiac electrophysiology problems. IEEE Trans. Biomed. Eng. 56, 1318–1330 (2009).
Arevalo, H., Plank, G., Helm, P., Halperin, H. & Trayanova, N. Tachycardia in post-infarction hearts: insights from 3D image-based ventricular models. PLoS One 8, e68872 (2013).
Plank, G. et al. From mitochondrial ion channels to arrhythmias in the heart: computational techniques to bridge the spatio-temporal scales. Phil. Trans A Math. Phys. Eng. Sci. 366, 3381–3409 (2008).
Gurev, V., Lee, T., Constantino, J., Arevalo, H. & Trayanova, N. A. Models of cardiac electromechanics based on individual hearts imaging data: image-based electromechanical models of the heart. Biomech. Model. Mechanobiol. 10, 295–306 (2011).
Deng, D. et al. Accuracy of prediction of infarct-related arrhythmic circuits from image-based models reconstructed from low and high resolution MRI. Front. Physiol. 6, 282 (2015).
Pashakhanloo, F. et al. Submillimeter diffusion tensor imaging and late gadolinium enhancement cardiovascular magnetic resonance of chronic myocardial infarction. J. Cardiovasc. Magn. Reson. 19, 9 (2017).
Ten Tusscher, K. H., Noble, D., Noble, P. J. & Panfilov, A. V. A model for human ventricular tissue. Am. J. Physiol. Heart Circ. Physiol. 286, H1573–H1589 (2004).
Decker, K. F. & Rudy, Y. Ionic mechanisms of electrophysiological heterogeneity and conduction block in the infarct border zone. Am. J. Physiol. Heart Circ. Physiol. 299, H1588–H1597 (2010).
Cabo, C. & Boyden, P. A. Electrical remodeling of the epicardial border zone in the canine infarcted heart: a computational analysis. Am. J. Physiol. Heart Circ. Physiol. 284, H372–H384 (2003).
Vigmond, E. J., Weber dos Santos, R., Prassl, A. J., Deo, M. & Plank, G. Solvers for the cardiac bidomain equations. Prog. Biophys. Mol. Biol. 96, 3–18 (2008).
Rodriguez, B., Li, L., Eason, J. C., Efimov, I. R. & Trayanova, N. A. Differences between left and right ventricular chamber geometry affect cardiac vulnerability to electric shocks. Circ. Res. 97, 168–175 (2005).
Rantner, L. J. et al. Three-dimensional mechanisms of increased vulnerability to electric shocks in myocardial infarction: altered virtual electrode polarizations and conduction delay in the peri-infarct zone. J. Physiol. 590, 4537–4551 (2012).
Bishop, M. J. et al. The role of photon scattering in optical signal distortion during arrhythmia and defibrillation. Biophys. J. 93, 3714–3726 (2007).
Cerqueira, M. D. et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105, 539–542 (2002).
Piccini, J. P. et al. Mode of induction of ventricular tachycardia and prognosis in patients with coronary disease: the Multicenter UnSustained Tachycardia Trial (MUSTT). J. Cardiovasc. Electrophysiol. 20, 850–855 (2009).
Buxton, A. E. Programmed ventricular stimulation: not dead. Circulation 129, 831–833 (2014).
Vadakkumpadan, F., Trayanova, N. & Wu, K. C. Image-based left ventricular shape analysis for sudden cardiac death risk stratification. Heart Rhythm 11, 1693–1700 (2014).
Ranjan, R. et al. Personalized MRI-based modeling predicts ventricular tachycardia vulnerability in patients receiving primary prevention ICDs. Circulation 134, A16247–A16247 (2016).
Boykov, Y. & Kolmogorov, V. An experimental comparison of min-cut/max-flow algorithms for energy minimization in vision. IEEE Trans. Pattern Anal. Mach. Intell. 26, 1124–1137 (2004).
Prakosa, A. et al. Dataset for Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. figshare https://doi.org/10.6084/m9.figshare.6613289 (2018).
Acknowledgements
This work was supported by the NIH Pioneer Award (DP1-HL123271) to N.A.T.
Author information
Authors and Affiliations
Contributions
A.P., H.J.A., D.D., H.A. and P.P.N. performed animal and human LGE-MRI scan segmentation and model creation. A.P., H.J.A., D.D. and N.A.T. designed the simulation protocols. D.D., H.J.A., A.P. and P.N. performed simulations of VT in all models. D.D., A.P., P.M.B., H.J.A. and N.A.T. analysed the data. A.P. developed the pipeline for model generation from MRI scans with ICD artefact. D.D. and A.P. adapted the automatic algorithm for determining the ablation tragets in the ventricles. H.H. provided the swine MRI and electrophysiological data, as well as input for the animal study. S.N. provided part of the human MRI scans (at Johns Hopkins), conducted the prospective studies at the University of Pennsylvania, and provided clinical guidance and input. J.C. provided the remainder of the human MRI scans and patient outcomes. A.P. developed the methodology for input of simulation data into the clinical CARTO mapping system. J.B., E.G., R.M. and R.R. developed and implemented the clinical protocols at the University of Utah. R.R. and F.H. recruited patients and conducted VT ablations for the prospective human study at the University of Utah. S.N. and D.C. recruited patients and conducted VT ablations for the prospective human study at the University of Pennsylvania. N.A.T. initiated the collaborations, designed and coordinated the studies with contributions from H.J.A. and H.H. (retroprospective swine and human studies), S.N. and R.R. (prospective human studies), and A.P. and S.N. (retrospective human with ICD study), and supervised all simulation studies. H.J.A., D.D., A.P., P.M.B., E.G. and R.R. generated figures, tables and videos. N.A.T. wrote the manuscript with input from A.P. and H.J.A. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
N.A.T. holds partial ownership of CardioSolv Ablation Technologies LLC. S.N. is a scientific advisor to CardioSolv Ablation Technologies LLC. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Supplementary Video 1
Initiation of VT for patient 2 in the retrospective human study of patients with ICDs.
Supplementary Video 2
Initiation of sustained VT in the heart model of the patient from the prospective human study at the University of Utah.
Rights and permissions
About this article
Cite this article
Prakosa, A., Arevalo, H.J., Deng, D. et al. Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat Biomed Eng 2, 732–740 (2018). https://doi.org/10.1038/s41551-018-0282-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0282-2
This article is cited by
-
Digital twins in medicine
Nature Computational Science (2024)
-
Digital twins for cardiac electrophysiology: state of the art and future challenges
Herzschrittmachertherapie + Elektrophysiologie (2024)
-
lifex-ep: a robust and efficient software for cardiac electrophysiology simulations
BMC Bioinformatics (2023)
-
A simple approach for image-based modelling of the heart that enables robust simulation of highly heterogeneous electrical excitation
Scientific Reports (2023)
-
Advanced Imaging Integration for Catheter Ablation of Ventricular Tachycardia
Current Cardiology Reports (2023)