Abstract
Continuous glucose monitors (CGMs), used by patients with diabetes mellitus, can autonomously track fluctuations in blood glucose over time. However, the signal produced by CGMs during the initial recording period following sensor implantation contains substantial noise, requiring frequent recalibration via finger-prick tests. Here, we show that coating the sensor with a zwitterionic polymer, found via a combinatorial chemistry approach, significantly reduces signal noise and improves CGM performance. We evaluated the polymer-coated sensors in mice as well as in healthy and diabetic non-human primates, and show that the sensors accurately record glucose levels without the need for recalibration. We also show that the coated sensors significantly abrogated immune responses, as indicated by histology, fluorescent whole-body imaging of inflammation-associated protease activity and gene expression of inflammation markers. The polymer coating may allow CGMs to become standalone measuring devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yach, D., Stuckler, D. & Brownell, K. D. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat. Med. 12, 62–66 (2006).
Zimmet, P., Alberti, K. G. M. M. & Shaw, J. Global and societal implications of the diabetes epidemic. Nature 414, 782–787 (2001).
Gabir, M. M. et al. The1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care 23, 1108–1112 (2000).
Zhuo, X. et al. The lifetime cost of diabetes and its implications for diabetes prevention. Diabetes Care 37, 2557–2564 (2014).
Clar, C., Barnard, K., Cummins, E., Royle, P. & Waugh, N. Self-monitoring of blood glucose in type 2 diabetes: systematic review. Health Technol. Assess. (Rockv.) 14, 1–140 (2010).
Kovatchev, B., Breton, M. & Clarke, W. Analytical methods for the retrieval and interpretation of continuous glucose monitoring data in diabetes. Methods Enzymol. 454, 69–86 (2009).
Boland, E. et al. Limitations of conventional methods of self-monitoring of blood glucose. Diabetes Care 24, 1858–1862 (2001).
Newman, J. D. & Turner, A. P. F. Home blood glucose biosensors: a commercial perspective. Biosens. Bioelectron. 20, 2435–2453 (2005).
Hovorka, R. Continuous glucose monitoring and closed-loop systems. Diabet. Med. 23, 1–12 (2006).
Shichiri, M., Yamasaki, Y., Kawamori, R., Hakui, N. & Abe, H. Wearable artificial endocrine pancreas with needle-type glucose sensor. Lancet 320, 1129–1131 (1982).
Hovorka, R. Closed-loop insulin delivery: from bench to clinical practice. Nat. Rev. Endocrinol. 7, 385–395 (2011).
Veiseh, O., Tang, B. C., Whitehead, K. A., Anderson, D. G. & Langer, R. Managing diabetes with nanomedicine: challenges and opportunities. Nat. Rev. Drug Discov. 14, 45–57 (2014).
Mastrototaro, J. J. The MiniMed continuous glucose monitoring system. Diabetes Technol. Ther. 2, 13–18 (2004).
Girardin, C. M., Huot, C., Gonthier, M. & Delvin, E. Continuous glucose monitoring: a review of biochemical perspectives and clinical use in type 1 diabetes. Clin. Biochem. 42, 136–142 (2009).
Gifford, R. Continuous glucose monitoring: 40 years, what we’ve learned and what’s next. ChemPhysChem 14, 2032–2044 (2013).
Rodbard, D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol. Ther. 18 (Suppl. 2), S23–S213 (2016).
Vaddiraju, S., Burgess, D. J., Tomazos, I., Jain, F. C. & Papadimitrakopoulos, F. Technologies for continuous glucose monitoring: current problems and future promises. J. Diabetes Sci. Technol. 4, 1540–1562 (2010).
Gerritsen, M. Problems associated with subcutaneously implanted glucose sensors. Diabetes Care 23, 143–145 (2000).
Nichols, S. P., Koh, A., Storm, W. L., Shin, J. H. & Schoenfisch, M. H. Biocompatible materials for continuous glucose monitoring devices. Chem. Rev. 113, 2528–2549 (2013).
Gerritsen, M., Jansen, J. A. & Lutterman, J. A. Performance of subcutaneously implanted glucose sensors for continuous monitoring. Neth. J. Med. 54, 167–179 (1999).
Novak, M. T., Yuan, F. & Reichert, W. M. Predicting glucose sensor behavior in blood using transport modeling: relative impacts of protein biofouling and cellular metabolic effects. J. Diabetes Sci. Technol. 7, 1547–1560 (2013).
Jadviscokova, T., Fajkusova, Z., Pallayova, M., Luza, J. & Kuzmina, G. Occurrence of adverse events due to continuous glucose monitoring. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 151, 263–266 (2007).
iPro2 User Guide (Medtronic MiniMed, 2017).
Boyne, M. S., Silver, D. M., Kaplan, J. & Saudek, C. D. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes 52, 2790–2794 (2003).
Ellison, J. M. et al. Rapid changes in postprandial blood glucose produce concentration differences at finger, forearm, and thigh sampling sites. Diabetes Care 25, 961–964 (2002).
Sylvain, H. F. et al. Accuracy of fingerstick glucose values in shock patients. Am. J. Crit. Care 4, 44–48 (1995).
McGarraugh, G. The chemistry of commercial continuous glucose monitors. Diabetes Technol. Ther. 11, S-17–S-24 (2009).
Wang, J. in Electrochemical Sensors, Biosensors and their Biomedical Applications (eds Zhang, X., Ju, H. & Wang, J.) 57–69 (Elsevier, New York, 2008).
Basu, A., Veettil, S., Dyer, R., Peyser, T. & Basu, R. Direct evidence of acetaminophen interference with subcutaneous glucose sensing in humans: a pilot study. Diabetes Technol. Ther. 18 (Suppl. 2), S243–S247 (2016).
Klueh, U., Frailey, J. T., Qiao, Y., Antar, O. & Kreutzer, D. L. Cell based metabolic barriers to glucose diffusion: macrophages and continuous glucose monitoring. Biomaterials 35, 3145–3153 (2014).
Klueh, U., Kaur, M., Qiao, Y. & Kreutzer, D. L. Critical role of tissue mast cells in controlling long-term glucose sensor function in vivo. Biomaterials 31, 4540–4551 (2010).
Onuki, Y., Bhardwaj, U., Papadimitrakopoulos, F. & Burgess, D. J. A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. J. Diabetes Sci. Technol. 2, 1003–1015 (2008).
Anderson, J. M. Biological responses to materials. Annu. Rev. Mater. Res. 31, 81–110 (2001).
Moussy, F. Implantable glucose sensor: progress and problems. In Proceedings of IEEE Sensors 270–273 (IEEE, 2002).
Meyers, S. R. & Grinstaff, M. W. Biocompatible and bioactive surface modifications for prolonged in vivo efficacy. Chem. Rev. 112, 1615–1632 (2012).
Vallejo-Heligon, S. G., Brown, N. L., Reichert, W. M. & Klitzman, B. Porous, dexamethasone-loaded polyurethane coatings extend performance window of implantable glucose sensors in vivo. Acta Biomater. 30, 106–115 (2016).
Klueh, U., Kaur, M., Montrose, D. C. & Kreutzer, D. L. Inflammation and glucose sensors: use of dexamethasone to extend glucose sensor function and life span in vivo. J. Diabetes Sci. Technol. 1, 496–504 (2007).
Vaisocherová, H. et al. Ultralow fouling and functionalizable surface chemistry based on a zwitterionic polymer enabling sensitive and specific protein detection in undiluted blood plasma. Anal. Chem. 80, 7894–7901 (2008).
Zhang, L. et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 31, 553–556 (2013).
Zhao, J. et al. Improved biocompatibility and antifouling property of polypropylene non-woven fabric membrane by surface grafting zwitterionic polymer. J. Memb. Sci. 369, 5–12 (2011).
Klueh, U., Antar, O., Qiao, Y. & Kreutzer, D. L. Role of vascular networks in extending glucose sensor function: impact of angiogenesis and lymphangiogenesis on continuous glucose monitoring in vivo. J. Biomed. Mater. Res. Part A 102, 3512–3522 (2014).
Englert, K. et al. Skin and adhesive issues with continuous glucose monitors: a sticky situation. J. Diabetes Sci. Technol. 8, 745–751 (2014).
Abbott Laboratories. FreeStyle Libre, flash glucose monitoring system. Abbott Libre https://www.freestylelibre.us (2018).
Bailey, T., Bode, B. W., Christiansen, M. P., Klaff, L. J. & Alva, S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol. Ther. 17, 787–794 (2015).
Hoss, U. & Budiman, E. S. Factory-calibrated continuous glucose sensors: the science behind the technology. Diabetes Technol. Ther. 19, S-44–S-50 (2017).
Bequette, B. W. Continuous glucose monitoring: real-time algorithms for calibration, filtering, and alarms. J. Diabetes Sci. Technol. 4, 404–18 (2010).
Doloff, J. C. et al. Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates. Nat. Mater. 16, 671–680 (2017).
Prichard, H. L., Schroeder, T., Reichert, W. M. & Klitzman, B. Bioluminescence imaging of glucose in tissue surrounding polyurethane and glucose sensor implants. J. Diabetes Sci. Technol. 4, 1055–1062 (2010).
Yang, W., Xue, H., Carr, L. R., Wang, J. & Jiang, S. Zwitterionic poly(carboxybetaine) hydrogels for glucose biosensors in complex media. Biosens. Bioelectron. 26, 2454–2459 (2011).
Yang, W. et al. The effect of lightly crosslinked poly(carboxybetaine) hydrogel coating on the performance of sensors in whole blood. Biomaterials 33, 7945–7951 (2012).
Reid, B. et al. PEG hydrogel degradation and the role of the surrounding tissue environment. J. Tissue Eng. Regen. Med. 9, 315–318 (2015).
Bratlie, K. M. et al. Rapid biocompatibility analysis of materials via in vivo fluorescence imaging of mouse models. PLoS ONE 5, e10032 (2010).
Vegas, A. J. et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol. 34, 345–352 (2016).
Yesilyurt, V. et al. A facile and versatile method to endow biomaterial devices with zwitterionic surface coatings. Adv. Healthc. Mater. 6, 1601091 (2017).
Lee, H., Dellatore, S. M., Miller, W. M. & Messersmith, P. B. Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430 (2007).
Lee, H., Rho, J. & Messersmith, P. B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 21, 431–434 (2009).
Kang, S. M. et al. One-step multipurpose surface functionalization by adhesive catecholamine. Adv. Funct. Mater. 22, 2949–2955 (2012).
Facchinetti, A., Sparacino, G. & Cobelli, C. Signal processing algorithms implementing the ‘smart sensor’ concept to improve continuous glucose monitoring in diabetes. J. Diabetes Sci. Technol. 7, 1308–1318 (2013).
Vallejo-Heligon, S. G., Klitzman, B. & Reichert, W. M. Characterization of porous, dexamethasone-releasing polyurethane coatings for glucose sensors. Acta Biomater. 10, 4629–4638 (2014).
Salesov, E., Zini, E., Riederer, A., Lutz, T. A. & Reusch, C. E. Comparison of the pharmacodynamics of protamine zinc insulin and insulin degludec and validation of the continuous glucose monitoring system iPro2 in healthy cats. Res. Vet. Sci. 118, 79–85 (2018).
Chen, X., Lawrence, J., Parelkar, S. & Emrick, T. Novel zwitterionic copolymers with dihydrolipoic acid: synthesis and preparation of nonfouling nanorods. Macromolecules 46, 119–127 (2013).
Acknowledgements
This work was supported by the Leona M. and Harry B. Helmsley Charitable Trust Foundation (2015PG-T1D063), Juvenile Diabetes Research Foundation (JDRF) (Grant 17-2007-1063) and National Institutes of Health (Grants EB000244, EB000351, DE013023 and CA151884), and through a generous gift from the Tayebati Family Foundation. J.C.D. was supported by JDRF postdoctoral fellowship (Grant 3-PDF-2015-91-A-N). J.O. is supported by the National Institutes of Health (NIH/NIDDK) R01DK091526 and the Chicago Diabetes Project. X.X. was supported by the 100 Talents Program of Sun Yat-Sen University (76120-18821104) and 1000 Talents Youth Program of China and acknowledges financial support from the National Natural Science Foundation of China (Grant No.51705543, 61771498 and 31530023) and Science and Technology Program of Guangzhou, China (Grant No. 20180310097). In addition, of extreme importance, the authors thank the Histology and Whole Animal Imaging cores for use of resources (Swanson Biotechnology Center, David H. Koch Institute for Integrative Cancer Research at MIT).
Author information
Authors and Affiliations
Contributions
X.X., J.C.D., V.Y., A.S. and D.G.A. designed experiments, analysed data and wrote the manuscript. X.X., J.C.D., V.Y., A.S., J.J.M., M.O., S.F., D.I., S.G., I.J., J.L., W.W., A.B. and K.A.W. performed experiments. X.X., J.C.D., V.Y., A.S., H.H.T., J.T., H.-j.C. and B.Y. performed statistical analyses of datasets and aided in the preparation of displays communicating datasets. R.L. and D.G.A. provided conceptual advice. R.L. and D.GA. supervised the study. All authors discussed the results and assisted in the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary discussion, figures and references.
Rights and permissions
About this article
Cite this article
Xie, X., Doloff, J.C., Yesilyurt, V. et al. Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer. Nat Biomed Eng 2, 894–906 (2018). https://doi.org/10.1038/s41551-018-0273-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-018-0273-3
This article is cited by
-
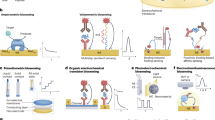
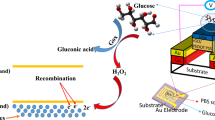
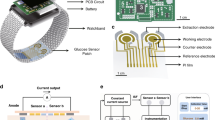
Interstitial fluid-based wearable biosensors for minimally invasive healthcare and biomedical applications
Communications Materials (2024)
-
Implantable Electrochemical Microsensors for In Vivo Monitoring of Animal Physiological Information
Nano-Micro Letters (2024)
-
Screening hydrogels for antifibrotic properties by implanting cellularly barcoded alginates in mice and a non-human primate
Nature Biomedical Engineering (2023)
-
Controlled swelling of biomaterial devices for improved antifouling polymer coatings
Scientific Reports (2023)
-
Anti-biofouling strategies for implantable biosensors of continuous glucose monitoring systems
Frontiers of Chemical Science and Engineering (2023)